1. Pure Substance
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chapter-2
Is Matter around us Pure
Is Matter around us Pure Introduction
We come across different substances like water that we drink; salt that we add in food for taste, milk that we drink as it provides us with calcium and other minerals, soaps that we use to wash clothes, paint that we use to colour walls and so on. These substances have different nature, different properties. They can be pure or there can be some impurities in them. Let us see what do we understand by the words pure or impure substance[12]
We come across different substances like water that we drink; salt that we add in food for taste, milk that we drink as it provides us with calcium and other minerals, soaps that we use to wash clothes, paint that we use to colour walls and so on. These substances have different nature, different properties. They can be pure or there can be some impurities in them. Let us see what do we understand by the words pure or impure substances[15]
We come across different substances like water that we drink; salt that we add in food for taste, milk that we drink as it provides us with calcium and other minerals, soaps that we use to wash clothes, paint that we use to colour walls and so on. These substances have different nature, different properties. They can be pure or there can be some impurities in them. Let us see what do we understand by the words pure or impure substances[20]
Pure substance
They are substances that are made up of a single type of particle. But there are certain characteristics that determine the purity of a substance. Let us see what all properties they possess.
Characteristics of a pure substance are as follows
1. They all have a uniform composition (homogeneous).
2. They can not be separated into their constituents.
3. They have fixed melting and boiling points.
4. They always have the same characteristic properties.
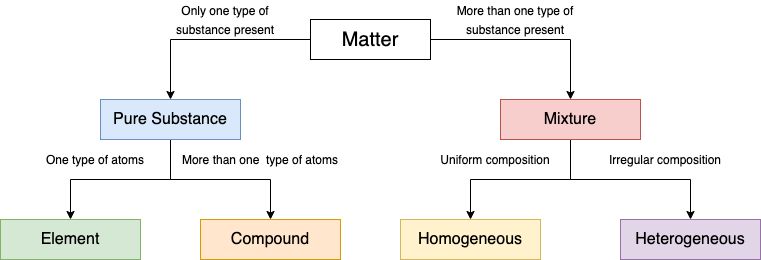
Two categories of substances fall under pure substance. They are as follows
1. Elements
2. Compounds
Element
As we know, an atom is the smallest particle that may or may not exist independently. These atoms unite together and form an element. This element can exist independently but it can not be broken into atoms as they are not visible. About 118 elements are known so far and still many discoveries are in the pipeline. Elements can be prepared artificially in lab, also by transmutation process. The elements having atomic number more than 92 are manmade and are called transuranic elements. An element is defined as a substance that can not be broken into simpler substances as it is formed of atoms and atoms can not be seen.
The characteristics of elements are as follows
1. They are made up of atoms
2. The physical & chemical properties of an element are due to the arrangement of atoms.
3. They can occur in nature in free or combined form.
4. They can be prepared artificially by nuclear reaction.
5. They can be solids, liquids (only 3) or gases (11) at normal room temperature.
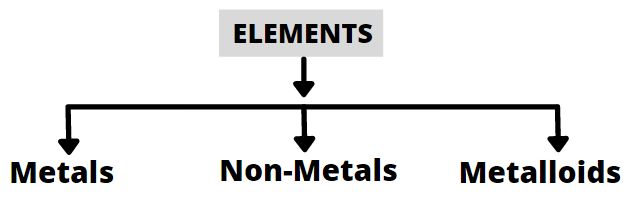
Metals
In daily routine, we use a lot of substances that fall in this category like the vehicles which are usually made up of iron, furniture, utensils and many more. Let us see their properties. Those substances which have a tendency to lose electrons and form positively charged species, that is cation are called metals.
Na – 1e- → Na+ (cation)
2, 8, 1 2, 8
Physical properties of metals are as follows
- They are malleable like Al(aluminum), Ag(silver) [ except alkali metals that is Na,K,Li etc]
- They are ductile like Al, Ag, etc.
- They are lustrous (except – Hg(mercury), Cs(cesium), Ga (gallium)) .
- They are hard (except Na & K ).
- They are good conductors of heat & electricity.
- They have high melting and boiling points [except Na, k, Ca they have low boiling and melting point] .
- They are Sonorous (that is when hit, they produce a sound).

Chemical Properties of metals are as follows
1. Reaction with oxygen
Metals react with oxygen to form oxides.
M + O2 → Metal oxides
All Metal oxides are basic in nature i.e. they turn red litmus blue but some metal oxides are amphoteric in nature (that is, they have acidic & basic nature) like Al2O3 (aluminum oxide) & ZnO (zinc oxide).
2. Reaction with dilute acids
Metals react with dilute acids to form a salt and H2 gas.
M + HX → MX + H2
Example: Na + HCl → NaCl + H2
The hydrogen gas, when comes in contact with air, burns with a popping sound.
Non-Metals
Non – Metals are those elements that always gain electrons and form anions (negatively – charged ions).
Example: Cl + e- → Cl- anion
2, 8, 7 2, 8, 8
Physical properties of non – metals are as follows
- They are liquid or gases (only one exists in liquid form – that is Bromine) except C, S, P, I that are solids.
- They are non – malleable and non – ductile because to make wires or sheets, we need to hammer them, but as they are brittle, they break.
- They are bad conductors of heat and electricity (like graphite).
- They have low melting and boiling point (except B, C which have high melting and boiling points).
- They are non sonorous.
- They are non lustrous (except graphite and iodine).

Chemical properties of non – metals are as follows
1. Reaction with oxygen
Non – metals react with O2 to form Non Metal oxide with respective formula as given below:
N + O2 → non metal oxide
They are acidic in nature and turn blue litmus red.
2. Reaction with dilute acids
Non metals do not react with die acids as they do not have sufficient electrons.
Metalloids
They are those which have properties similar to metals and non-metals. Few elements exist as Noble gases. They are those which are stable elements as they have a stable configuration and generally exist free in nature as they do not need to combine with other elements because they have a stable electronic configuration. The noble gases are He (helium), Ne (neon), Ar (Argon), Kr (krypton), Xe (xenon) and radon.

Compounds
We use so many compounds like salt, water, fertilizers, etc. Let us learn about their properties. They are formed when 2 or more elements combine in a fixed whole number ratio. Example: H2O (water), NaCl (sodium chloride), etc.

Characteristics of compounds are as follows
- They are homogenous.
- The properties of a compound are entirely different from its constituents.
- The constituents can not be separated by physical methods.
- They have fixed properties like Melting point and Boiling point.
- The formation of compound is accompanied by energy changes.
2. Impure Substance
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Impure substance
The substances that are formed by different kinds of particles are called impure substances. For example : Mixtures are impure substances.
Mixtures
You must have eaten pakoras at home. Have you noticed how your mother prepares it. She makes a batter containing besan, water, spices, etc. These constituents can be added in any ratio. In all ratios, they will form pakoras but the difference will be that sometimes, they will be hard to touch or the batter may be too loose to fry.
Mixtures: They are formed when two or more substances are simply mixed in any ratio and are not chemically combined with each other.

Characteristics of a mixture are as follows
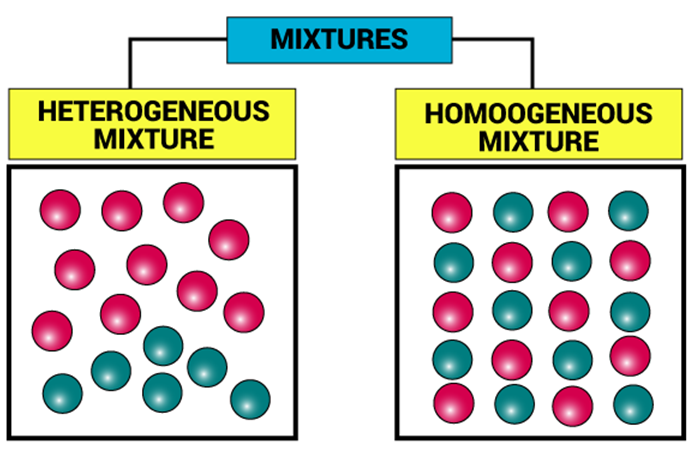
- They can be homogeneous or heterogeneous in nature, that is, the constituents can be seen to have visible boundaries or they may appear to mix thoroughly.
- The properties of a mixture are the same as that of its constituents.
- The constituents can be separated by physical methods.
- Their formation does not require or release energy as there is no bond formation or breakage involved.
- The properties of mixtures like melting point & boiling point are not fixed.
Types of Mixtures
Solutions
We all like drinking sweet lemon water in summers as it gives a cooling effect. It is made of lemon, water, ice, salt and sugar. These all components when mixed, form sweet lemon water mixture which is also called a solution. These components can be separated by using different techniques. Let us learn what exactly solutions are and their types.
A Solution is a homogenous mixture of two or more substances. To form it, we can add 2 or more components. These components can be in any ratio but are simply mixed, that is not chemically combined. Generally, two components of solutions are seen and are called ‘Solute’ and ‘Solvent’.
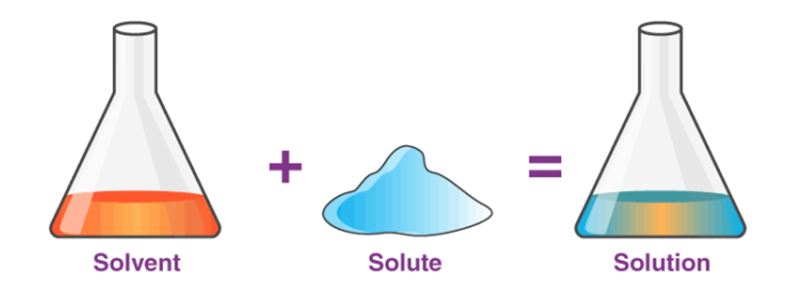
Solute
It is the constituent which is present in a comparitively lesser amount and gets dissolved in the solvent.
Solvent
It is the constituent present in more amount and it has the ability to dissolve the solute in it. If you dissolve salt in water, then the salt is in lesser quantity and it gets dissolved. So, here solute is salt and solvent is water
Characteristics of solutions are as follows
- They are homogeneous.
- Their Composition can vary.
- The size of particles is very small.
- They do not scatter light.
- They can be separated by physical methods.
Types of solutions
1. On the basis of dissolving nature of liquids
You must have noticed that when you dissolve sugar or salt in water, it just vanishes after a few seconds or a minute. The reason is that it gets mixed in water. But if we add oil in water, it does not vanish and is seen floating on its surface. It is due to this reason that some substances can mix into each other and some do not. Let us learn about it in detail
Miscible, immiscible & partially miscible solutions
Miscible liquids
The liquids that completely mix into each other to form a solution are miscible liquids. For example: alcohol when added to water gets completely mixed.

Partially miscible liquids
The liquids which can dissolve in another liquid only up to some extent to form a solution are partially miscible liquids. For example: ethylene glycol in chloroform.
Immiscible liquids
The liquids which do not mix into each other are immiscible liquids. For example: oil & water.

2. On the basis of nature of solvent
We have seen that it’s not only water in which we can dissolve substances, we can make use of other substances as well. For example- carbon tetrachloride, Benzene, alcohol, etc. and many more reagents. So, we have another classification based on the nature of solvent.
Aqueous and Non aqueous solutions
Aqueous
The solution in which the solvent is water is an aqueous solution. Example: salt solution
Non-aqueous solutions
The solution in which the solvent is other than water is a non-aqueous solution. Example: alcohol, benzene etc.
3. Classification on the basis of solubility power of solvent
You must have seen that if you take one glass of water at room temperature and you add 1 spoon of sugar to it, it dissolves. But if you keep on adding sugar to the same solution, a point will be reached when it stops dissolving sugar in it and the sugar starts getting deposited at the bottom. The reason being that each solvent has some solubility power and it can dissolve only up to that limit. Let us study about it.
Saturated, unsaturated and supersaturated solutions
Saturated Solution
The solution that dissolves as much solute as it is capable of dissolving is a saturated solution.
Unsaturated solution
The solution in which more quantity of solute can be dissolved without increasing its temperature is an unsaturated solution.
Supersaturated
The solution in which the solvent dissolves an amount of solute greater than its solubility. It is formed at high temperature and then slowly cooling it to lower its solubility.
Solubility
Is the amount of solute that can be dissolved in a given amount of solvent at a particular temperature.
Factors affecting solubility
- Nature of solute.
- Nature of solvent.
- Temperature.
Please Note:
- On lowering temperature, solubility of liquids & solid decreases & solubility of gas remains unaffected.
- On increasing pressure, solubility of gas increases & for solid and liquid, it remains unaffected.
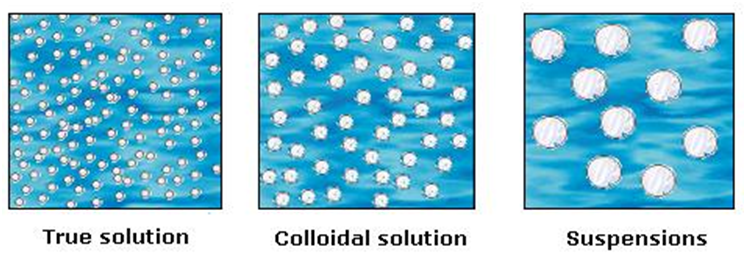
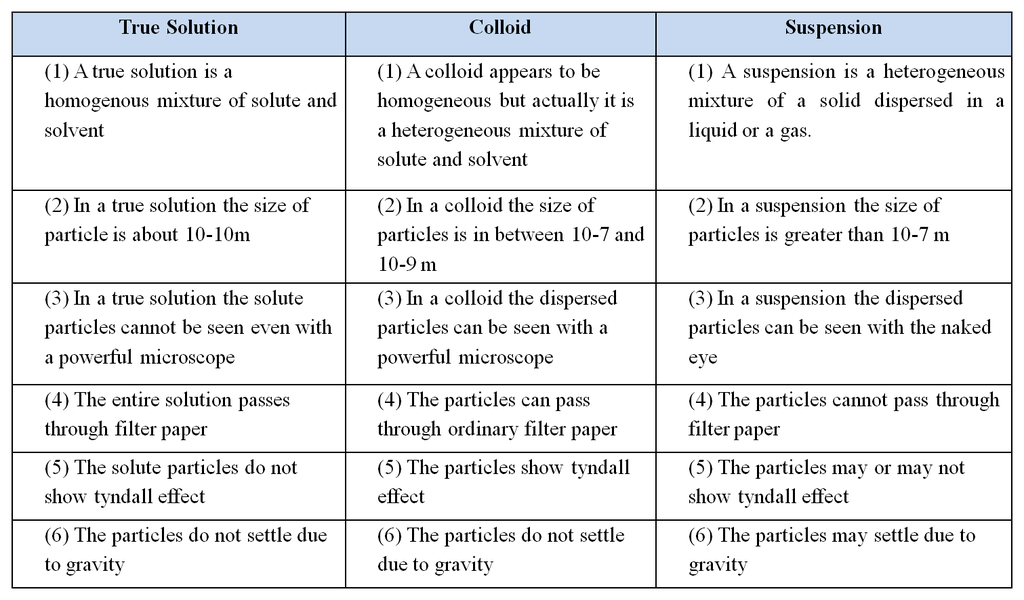
4. Classification on the basis of size of solute particles
You must have made a solution of sand in water, sugar in water and milk. They don’t look the same. Let us predict the nature of these solutions
Difference between a true solution, colloid and a suspension
3. Separation of Mixtures
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Separation of Mixtures
1. To obtain colored component (dye) from Ink
Materials Required: Watch glass, Ink (blue/ black), Beaker, Stand, Burner
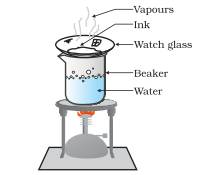
Procedure
1. Take a beaker and fill it half with water.
2. Take few ml of ink (Blue/ black) in the watch glass and place it on the mouth of the beaker.
3. Start heating the beaker and observe.
4. Heating is continued as long as the evaporation is taking place
5. Heating is stopped when no further change can be noticed on the watch glass
Observation
1. Evaporation taking place from the watch glass can be seen
2. Residue is left on the watch glass
Conclusion drawn
1. It can be concluded that,” Ink is not a pure substance but it is a mixture of dye in water which can easily be separated by evaporation method”.
2. Ink is not a single substance
3. Evaporation taking place from the watch glass containing ink.
Applications of evaporation process are as follows
1. This method can be used to separate the volatile component (solvent) from its non-volatile solute.
2. To obtain common salt from sea water. Sea water is trapped in small shallow pits (called lagoons) and is allowed to stand there for a few days. During this time the heat of the Sun evaporates water in these pits leaving behind the solid salt. This salt is further purified by crystallization method which you will study in the later part of this chapter.
2. To separate cream from milk by using centrifugation method.
Materials Required: Full cream milk, Centrifuging machine/milk churner, Jug test-tubes
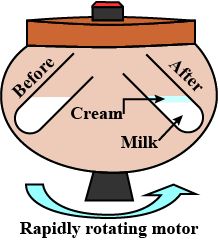
Procedure
1. Take un boiled cold milk in two test-tubes and place these test-tubes in a centrifuging machine .
2. Centrifuge it at high speed by using a hand centrifuging machine for two minute and observe.
Note: If you don’t have centrifuging machine in your school then you can do this activity by using a milk churner used in the kitchen.
Observation:
Cream floating on the milk can be seen
Conclusion drawn:
When milk is rotated at high speed, then the suspended lighter particles (fats and protein molecules) bind with each other forming ‘cream’ and ‘skimmed milk’. The cream being lighter, floats over the skimmed milk which can be removed easily.
Applications of centrifugation method are as follows
1. This technique is used in washing machines to squeeze out water from wet clothes and make them dry.
2. This technique is used in diagnostic laboratories for testing cholesterol lipids in blood, ESR (Erythrocyte Sedimentation Rate) and various other types of tests.
3. It is used in dairies for separating cream from milk and butter from curd.
3. To separate kerosene oil from water by using a separating funnel.
Materials Required: Separating funnel, Iron stand, kerosene oil, Beaker, Water.
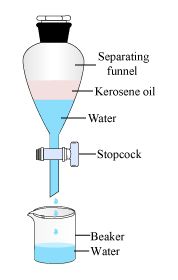
Procedure
1. A mixture of kerosene oil and water is taken in a separating funnel. The separating funnel is clamped on an iron stand.
2. The mixture is allowed to stand so that it forms two distinct layers.
3. A clean beaker is placed below and the stop cock is opened slowly so that water falls into the beaker.
4. Close the stop- cock of the separating funnel as the oil reaches the stop cock.
Observation:
Kerosene oil being lighter forms the upper layer.
Conclusion drawn:
When a liquid mixture containing two immiscible liquids is taken in a separating funnel, then the liquid layers stand one above the other. The liquid layer with greater density forms the lower layer whereas the lighter liquid (having lower density) forms the upper layer.
Applications of this method are as follows
1. They separate mixture of oil and water.
2. In the extraction of iron from its ore, the lighter slag is removed from the top by this method to leave the molten iron at the bottom in the furnace.
4. To separate a mixture of common salt and ammonium chloride
Materials Required: China dish, Tripod stand, Mixture of common salt and ammonium chloride, Glass funnel, Cotton, Burner.
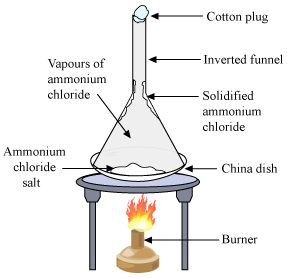
Procedure
1. Take the mixture of sand and ammonium chloride in a china dish.
2. Cover the china dish with an inverted glass funnel and place it on a tripod stand.
3. Put a loose cotton plug in the opening of the funnel so as to prevent the escape of ammonium chloride vapours.
4. Heat the china dish on a low flame and observe.
Observation
1. White fumes (vapours) of ammonium chloride can be seen coming out of the mixture.
2. These white fumes start depositing as white solid, on coming in contact with the cold, inner walls of the funnel.
3. Sand salt is left behind in the china dish.
Conclusion drawn:
Ammonium chloride, being a volatile substance, changes into white vapours easily which deposit on the cold inner wall of the funnel. This ammonium chloride obtained and is called as Sublimate. Some other examples of solids which sublime are: Iodine, camphor, naphthalene and anthracene.
5. To separate the dyes (colored components) present in black ink.
Materials Required: Strip of filter paper, Water soluble ink ( sketch pen or fountain pen), Large size glass jar with lid, Cotton, Thread.
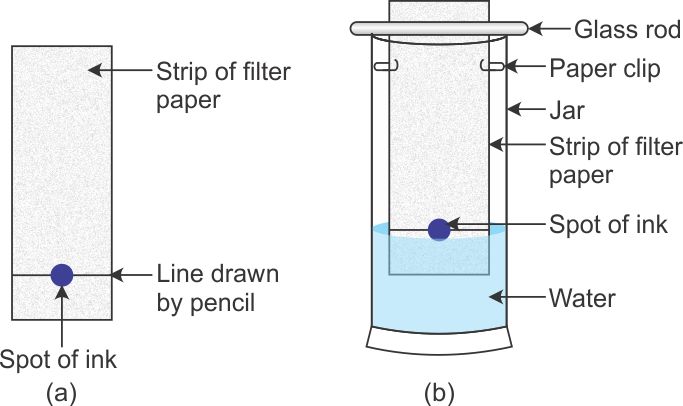
Procedure
1. Take a thin strip of filter paper (25cm x 5cm approx).
2. Draw a line on it using a pencil, approximately 3cm above the lower edge.
3. Put a small drop of water soluble black ink from a sketch pen or fountain pen) at the centre of the line. Let the ink dry.
4. Attach the paper strip on the thread with the help of cello tape.
5. Lower the filter paper strip into a large size gas jar in such a way that the drop of ink on the paper is just above the water level.
6. Adjust the thread and fix it on the sides of gas jar with the help of cello tape.
7. Cover the gas jar with a lid and leave it undisturbed.
8. Watch carefully as the water rises up on the filter paper.
9. Remove the filter paper strip, dry it and observe.
Observation:
Three colored spots can be seen on the filter paper strip.
Conclusion:
The given sample of black ink has three different dyes mixed in it.
6. To separate a mixture of two miscible liquids (acetone and water).
Materials Required: Distillation flask, Thermometer, Condenser, Beaker, Iron stand, Mixture of acetone and water.
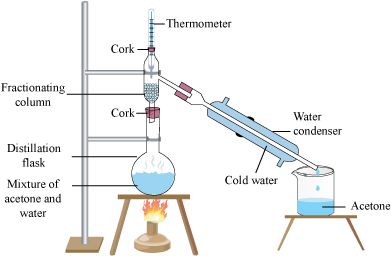
Procedure
1. Take the mixture in a distillation flask. Fit the flask with a thermometer.
2. Arrange the apparatus as shown in the figure.
3. Heat the mixture slowly, keeping a close watch at the thermometer and observe what happens.
Observation
1. The vapours of acetone can be seen rising up in the distillation flask with the increase in temperature.
2. When the temperature rises above 60o, the acetone gets vapourized and forms vapours in the flask.
3. These vapours get condensed in the condenser and can be collected (as pure liquid distillate) from the condenser outlet.
Result
Acetone can be collected in the beaker from the condenser outlet while water is left behind in the distillation flask.
7. To obtain crystals of pure copper sulphate salt from an impure sample by crystallization method.
Materials Required: Impure sample of copper sulphate, Beaker, China dish, Glass rod.
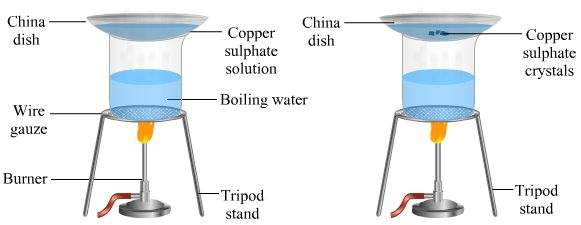
Procedure
1. Take about 5 grams of impure sample of copper sulphate in a china dish.
2. Dissolve it in minimum amount of water.
3. Filter the copper sulphate solution to remove the insoluble impurities.
4. Heat the copper sulphate solution gently on a water bath until it becomes saturated and reaches its crystallization point.
5. Crystallization point can be checked by taking some solution on a glass rod and waving it in the air. When a solid film (small crystals) is formed on the glass rod, then further heating is stopped. This indicates that the solution has been concentrated to crystallization point.
6. Place the china dish on the table after covering it with a watch glass. Leave it undisturbed at room temperature to cool slowly for a day and observe thereafter.
Observation
Crystals of copper sulphate can be seen in the china dish along with the mother liquor (the residual liquid which is left after crystallization).
Conclusions drawn
Hot and concentrated solution of any pure substance forms crystal on cooling gradually.
Result:
Pure crystals of copper sulphate are separated from the mother liquor. These crystals are dried further between the folds of a filter paper or on a porous plate.
8. Purification of surface water
The purification of surface water can be done through following steps
Sedimentation → loading → filtration → chlorination.
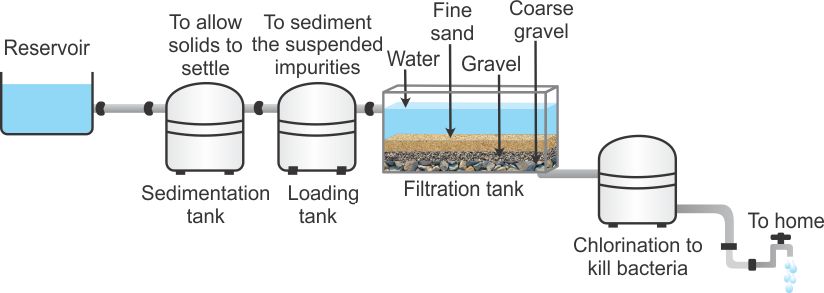
1. Sedimentation: The river water is pumped into a series of sedimentation tanks where it is allowed to stay for a day. Heavy particles of clay and other impurities settle down at the bottom due to the effect of gravity. The supernatant water is then sent to another settling tank.
2. Loading: In next chamber, water is treated with chemicals, i.e. alum and lime to get further settling of impurities.
3. Filtration: The clear water from the second tank is pumped into filtration tank where sand and gravel filter completely remove the suspended impurities.
4. Chlorination: The clear water is chlorinated with a calculated amount of chlorine in the chlorination tank. This process kills harmful bacteria and germs and provides safe drinking water.
4. Physical And Chemical Changes
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Physical and chemical Change
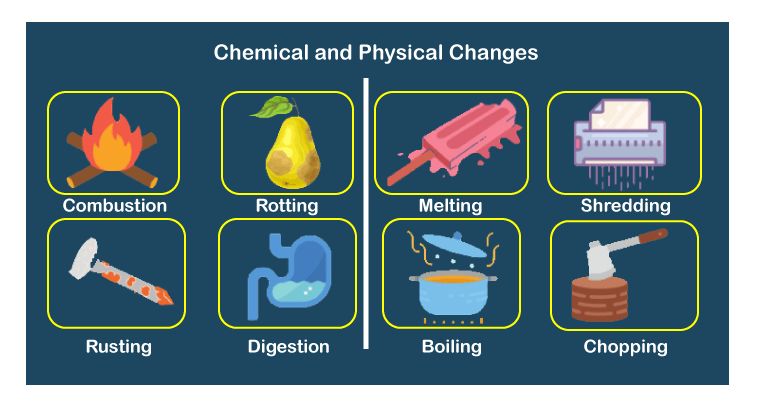
We observe different kind of changes around us like if you forget to keep the milk inside the fridge in summers it gets rancid, likewise when you keep ice out of the refrigerator, you observe the water around it and finally the ice gets converted into water. These all are changes that are taking place around us. But some changes can be reversed like water can be converted back to ice but rancid food item can not be made fresh again. Let us learn in detail about these changes.
1. Physical change
2. Chemical change


 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
