- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Diffusion and Osmosis
Diffusion and Osmosis
Diffusion: It is the movement of any substance from higher concentration to lower concentration. Have you noticed the fragrance of your favorite food at your home when it surprisingly cooked for you by your mom. This is due to the process diffusion. When it is being cooked in kitchen its fragrant molecules from kitchen start moving out into the kitchen’s surroundings and with time get dispersed in the air. The diffusion is seen in solids, liquids and gases but the rate of diffusion is faster in gases. This is because gas particles are energetic as they possess high kinetic energy. The movement of gases in and out from the cell occurs by diffusion
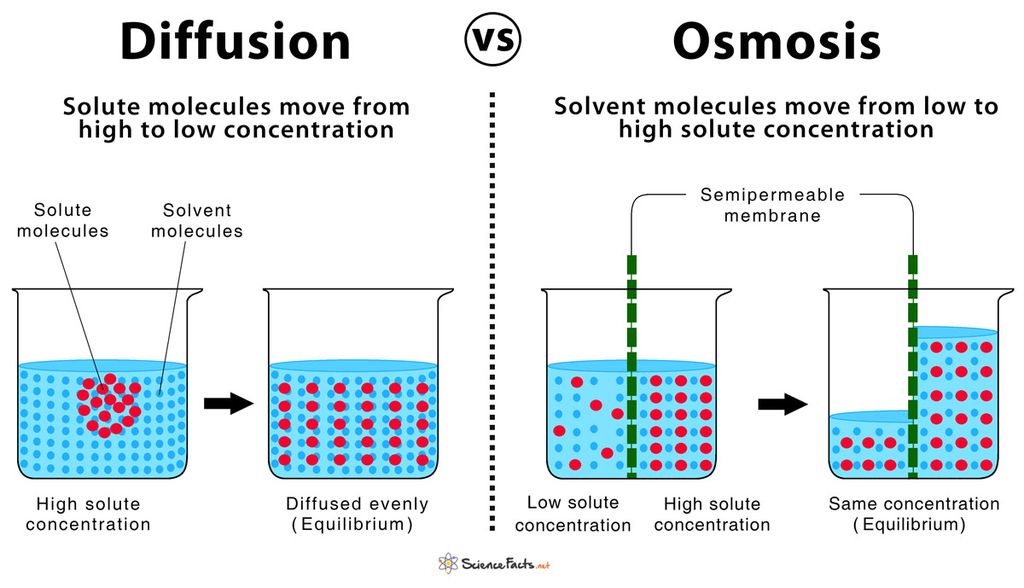
Osmosis: It is the movement of pure liquid from higher concentration to lower concentration across the semipermeable membrane . For example: if you take two flasks and fill one with low sugar so lution and other flask with highly concentrated sugar solution. Both the beakers are separated by a semipermeable membrane. Then we will notice that the water will move from the beaker where it is more to the side where it is less. The membrane allows only water to pass through it not sugar as it is semi-permeable that is selectively permeable.
Types of solutions
We can make three different types of solutions that is :
1. Hypotonic solution – The solution that has higher water concentration.
2. Hypertonic solution- The solution that has less water concentration.
3. Isotonic solution-The solution that has the same concentration of water as in cell.
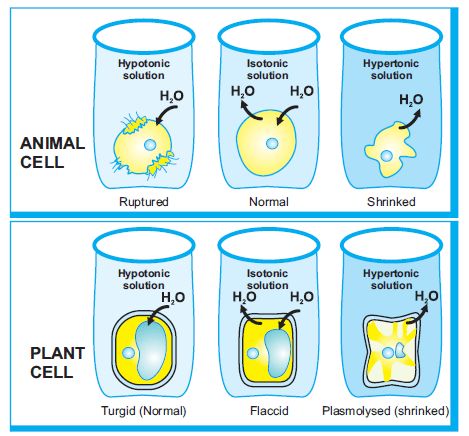
Now let’s do an activity in which we will be placing a cell say red blood cell in three different solutions. Let’s see what happens:
Cells placed in different types of solutions
This is because when it is placed in a hypotonic solution, the water from solution moves into cell (endo-osmosis) due to which cell starts swelling up .The fully swollen cell in which the protoplasm almost touches the cell membrane is called Turgid cell.
Have you seen the red blood cell kept in hypertonic solution looks flaccid This is because when it is placed in a hypertonic solution, the water from cell moves into solution (exo-osmosis) occurs due to which cell starts shrinking up .The cell whose protoplasm almost gets shrunk is called flaccid cell.
Have you seen the cell in isotonic solution It remains as such. Nothing happens. The reason being the concentration of cell and a solution is the same.
Q. Have you noticed that sometimes when you forget to water plants especially in summers they droop, dry and ultimately they are about to die .
A. This is because the water from the plant is moving out due to transpiration. So, when there is no water left in them they start to droop. This basically occurs due to exo-osmosis. But when it is watered again, the endo osmosis occurs and it again become turgid .this is called plasmolysis (shrinkage) and deplasmolysis (again becoming healthier).

 Vaishnav Publication
Vaishnav Publication
 ACERISE INDIA
ACERISE INDIA
