- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
How do We Control Fire?
We can control the fire by one or more of the following:
- Removing the fuel
- Cutting off the air supply (or oxygen supply)
- Cutting off heat or lowering the temperature of the fuel
- Fire Brigade Stations: In case of fire, fire brigades will extinguish the fire by sprinkling the water on the affected areas. The water will bring down the temperature below its ignition temperature. As a result, fire will stop spreading.
- Fire Extinguisher: Water is the most common fire extinguisher. But, it works only on things like wood, paper, etc. However, in case the fire is caught on electrical things then, water being a good conductor of electricity will destroy that equipment. Even water is not good in case of fires due to oil, petrol, etc. In that case, Carbon dioxide is the best extinguisher. This cuts off the air supply and t brings down the temperature below the ignition temperature as a result fire gets extinguished.
- Use of Blankets: if a person catches the fire, then blankets can be used to extinguish the fire.
- Forest Fires: when the temperature rises too high then the regions having dry grasses will catch the fire. This fire spreads rapidly from grasses to trees and eventually entire forest is on fire. And it is difficult to manage such fires.

How do fire extinguishers work?
Fire extinguishers are devices used to put out fires. They either cut off the air supply to fire or cool off the fuel (below the ignition temperature) or both.
The three main types of fire extinguishers are:
1. ‘Dry Powder’ Fire Extinguisher: This type of fire extinguisher contains a mixture of baking soda (sodium bicarbonate) and sand. When you throw it over the fire, the baking soda decomposes by its heat to produce carbon dioxide. Since CO2 is heavier than air, it descends to envelop the burning flame and cuts off its contact with air (and the oxygen supply).
2NaHCO3 → Na2CO3 + H2O + CO2
2. ‘Soda-acid’ Fire Extinguisher: This fire extinguisher is a metallic cylinder that contains the sodium bicarbonate (NaHCO3) solution. At the bottom of the cylinder, the concentrated sulphuric acid (H2SO4) is placed in a thin sealed glass tube. A fixed wire gauze surrounds this tube. Below the tube, a plunger is placed with its sharp end touching the thin glass tube. On the top of the cylinder, there is a nozzle that is sealed with wax.
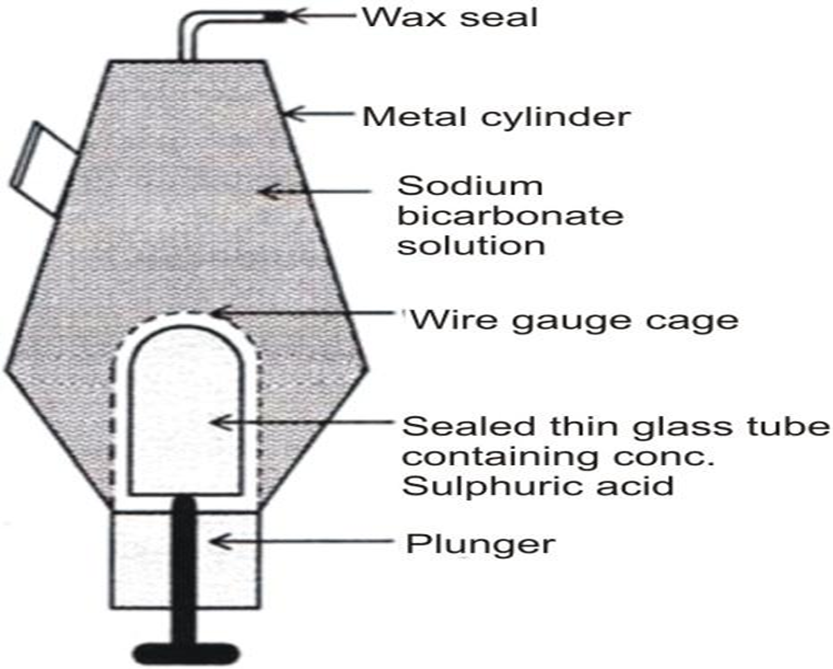
When the plunger hits against the floor, its sharp end breaks the thin glass tube and the acid inside it reacts with sodium bicarbonate to produce carbon dioxide. CO2 forces the wax seal open and rushes out of the nozzle to put out the fire in the direction where the nozzle is pointed.
3. ‘Foam-type’ Fire Extinguisher: Like the soda-acid fire extinguisher, it uses sodium bicarbonate solution. However, in this case, a substance called Saporin or Turkey Red Oil is added to the solution to produce foam along with the gas from the nozzle. Since this foam is lighter than oil, it floats on the surface of the oil and cuts off its air supply. Hence, it is very effective in putting out oil fires.
When should we use (or not use) water to extinguish the fire?
When wood, paper and clothes are on fire, we can use water to extinguish them. Water lowers the temperature of burning material below ignition temperature and thus, the fire stops burning. We should not use water when electrical equipment is on fire as water may conduct electricity and give a shock to people dousing the fire. Also, it should not be used when oil or petrol catches fire as water is lighter than oil and petrol and sinks down. Oil and petrol keep floating on the top and keep burning.
What should we do when electrical equipment or inflammable materials (like petrol) catch fire?
Carbon Dioxide is the best fire extinguisher in such cases. CO2 is heavier than oxygen and hence, covers the burning material like a blanket and cuts off its oxygen supply. Also, it does not harm the electrical equipment. CO2 can be stored as a liquid in cylinders at high pressure. When it is released, it immediately expands, cools down, and envelopes the fire - bringing down the temperature of the fuel. One can also pour dry chemicals like sodium bicarbonate (or baking soda) or potassium carbonate on the fire as they release CO2 near a fire.

 PathSet Publications
PathSet Publications
