- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
Electroplating
- Electroplating is a process in which layer of metal is deposited on another material with the help of electricity.
- Electroplating is used in many industries for depositing a layer of metal with desired characteristics on another metal.
- Different metals used for electroplating are Nickel, Copper, Gold Silver, Tin, Brass, Zinc, Chromium and Platinum.
Process of electroplating
- In order to conduct electroplating right electrodes and electrolytes must be chosen so that metal can deposit over a material.
- For instance, if we want to deposit copper on a material we need an electrolyte that contains copper in it. Similarly, if we need gold on a material we need an electrolyte that contains gold in it.
- Also, we should make sure that the electrode that we are choosing is completely clean.
- The electrodes used are made up of different materials. One of the electrodes is of the same metal of which the electrolyte solution is. The second electrode needs to be the material on which we want to coat another metal.
- For instance, in case we want to plate copper upon brass, one electrode should be of Copper and the other electrode should be of Brass and the electrolyte solution should be any salt that contains copper in it, for example, copper sulphate solution. Consider the diagram given below that describes the process of electroplating of copper.
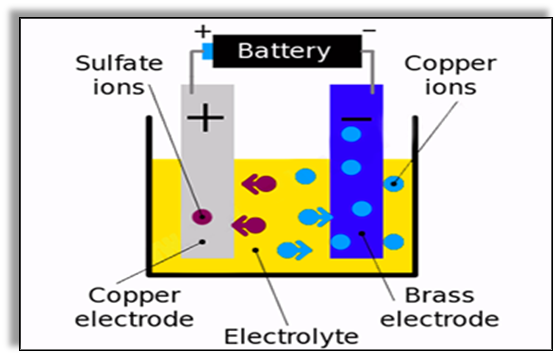
- Out of these two electrodes the copper electrode acts as the anode (positive electrode) and the brass electrode acts as the cathode (negative electrode).
- When electricity is passed through the solution, the copper sulfate breaks down into its ions.
- The copper ions (they have a positive charge) get attracted by the brass electrode while the sulphur ions being negatively charged move towards the copper electrode.
- As a result, copper starts depositing on the brass electrode.
- The process of electroplating takes some time to complete.
- The amount of time that it will take depends upon the strength of the current that is being passed through the circuit and also upon the concentration of the electrolyte.
- As these two are increased the speed of the electroplating process also increases.
Applications of Chemical Effects of Electric Current
Applications of Electroplating

- Medical equipment is made up of nickel which is harmful to the human body hence to avoid it from coming in contact with our body a coating of platinum or gold is applied on the surface of the nickel.
- Many kitchen equipments, bath taps, parts of cars, etc. are covered with chromium coating. Chromium is an expensive metal hence the objects are created with the cheaper metal and chromium coating is provided. Thus, to bring a shining over the objects and prevent them from corrosion chromium coating is used.
- Jewelry makers often make ornaments of less expensive metals and provide a coating of gold or silver upon them.
- The tin cans that are used to store food are actually made up of iron and have a coating of tin on them. Iron can easily react with food and spoil it, however, tin prevents the food from getting reacted with iron and therefore helps in preventing it from getting spoiled easily.
- Bridges and various parts of automobiles are made up of iron because it provides strength. However in order to prevent iron from getting rusted a coating of zinc is provided over it. This method is also called galvanization of iron.
Other applications of Chemical Effect of Electric Current
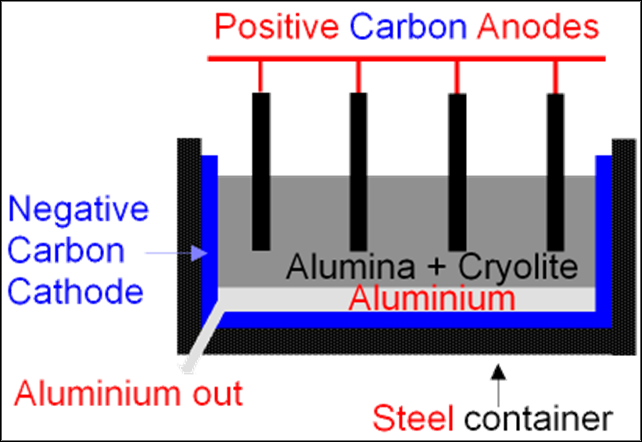
1. Extracting metals from their ores
Pure metals are extracted from metal ores with the process of electrolysis. Electricity is passed through the metal ores and they get broken down into an ionic lattice and thus the metal is obtained separately. For example, metals like aluminum, magnesium, potassium, sodium and calcium are obtained from their ores in this way.
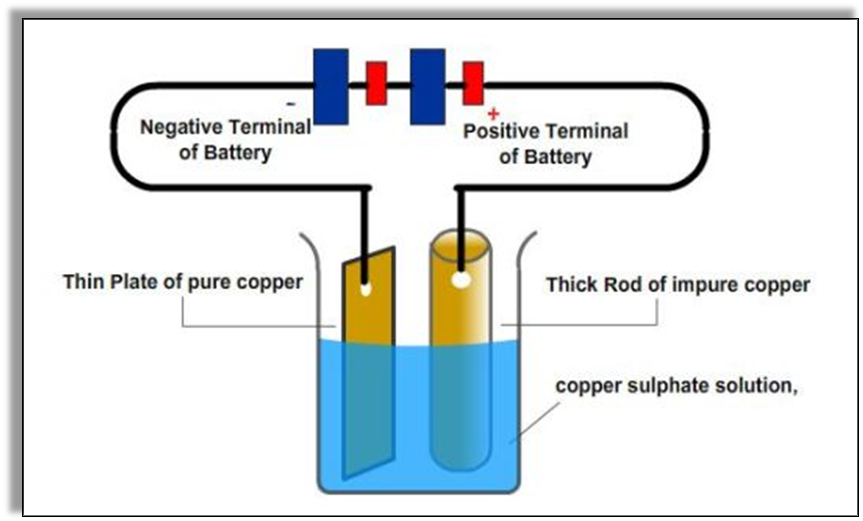
2. Purification of Metals
The method of electrolysis is also used to purify metal by separating it from the impurities. The impure metal is used as an anode which first dissolves in the electrolyte solution and then deposits on the cathode in the pure form. The impurities of the metal remain in the electrolyte solution only. Metals like aluminum, zinc and copper are purified in this way.
3. Production of Compounds
The electrolysis method is used for the production of some compounds. For example, sodium hypochlorite
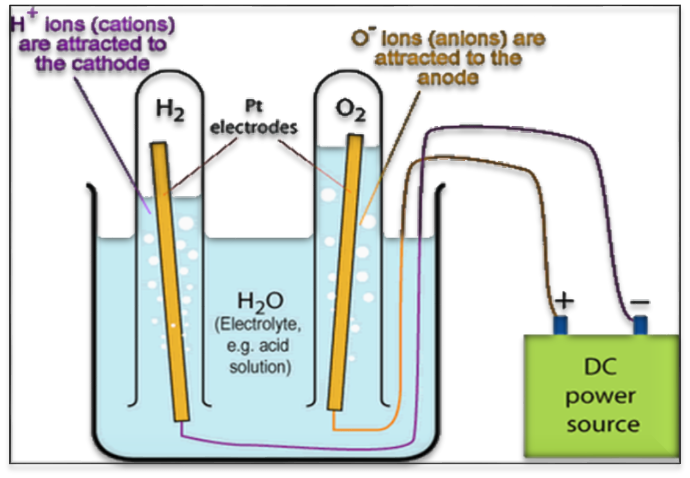
4. Decomposition of Compounds
The electrolysis method is also used to decompose a compound into its constituents. For example, water can be decomposed using the process of electrolysis to obtain hydrogen and oxygen.

 PathSet Publications
PathSet Publications
