- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
Chemical Properties of Metals and Non-metals
Reaction of Metals with Oxygen:
When metals react with oxygen, they form metal oxide.
Metal + O2 → Metal Oxide
For Example:
1. Copper + Oxygen → Copper Oxide (black)
2Cu + O2 → 2CuO
2. Aluminium + Oxygen → Aluminium Oxide
4Al + 3O2 → 2Al2O3
3. Magnesium + Oxygen → Magnesium Oxide
2Mg + O2 → 2MgO
The reactivity of metals with oxygen differs. For Example:
- Sodium and Potassium react so vigorously with air that they catch fire when they are kept in open. Hence, they are immersed in kerosene to store them.
- The surfaces of Magnesium, Aluminium, Zinc and Lead are covered with a thin layer of oxide to make sure that they do not get oxidized anymore.
- While iron does not burn when heated, iron filings burn vigorously.
- The copper surface is coated with a thin layer of black copper oxide to prevent the process of further oxidation from taking place.
- Gold and silver do not react with Oxygen.
Amphoteric Oxides: Some metal oxides react both with acids and bases to produce salts and water. Such metal oxides are called amphoteric oxides. For example:
Aluminum Oxide + Hydrochloric Acid → Aluminium Chloride + Water
Al2O3 + 6HCl → 2AlCl3 + H2O
Aluminum Oxide + Sodium Hydroxide → Sodium Aluminate + Water
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Reaction of Metals with Water
Metal + (cold) Water → Metal hydroxide + Hydrogen
Metal + Stream → Metal oxide + Hydrogen
For Example:
Sodium + Water → Sodium Hydroxide + Hydrogen + Heat
2Na + 2H2O → 2NaOH + H2 + Heat
Calcium + Water → Calcium Hydroxide + Hydrogen
Ca + 2H2O → Ca(OH)2 + H2
Magnesium + Water → Magnesium Hydroxide + Hydrogen
Mg + 2H2O → Mg(OH)2 + H2
Aluminium + Water → Aluminium Oxide + Hydrogen
2Al + 3H2O → Al2O3 + 3H2
Iron + Water → Iron Oxide + Hydrogen
3Fe + 4H2O → Fe3O4 + H2
The reactivity of metals with water differs. For Example:
- Sodium, Potassium and Calcium react with cold water.
- Magnesium reacts slowly with hot water to form slightly soluble magnesium hydroxide.
- Aluminum, Zinc and Iron react with steam.
- Lead, Copper, Silver and Gold do not react with water.
Note: Calcium and magnesium float on water as bubbles of hydrogen stick to their surface.
The reaction of Metals with Solutions of Other Metal Salts
Metal A + Salt Solution of Metal B → Salt Solution of Metal A + Metal B
More reactive metals replace less reactive metals.
Less reactive metals cannot replace more reactive metals.
For Example:
Iron + Copper Sulphate → Iron Sulphate + Copper
Fe + CuSO4 → FeSO4 + Cu
The reactivity series is given below:
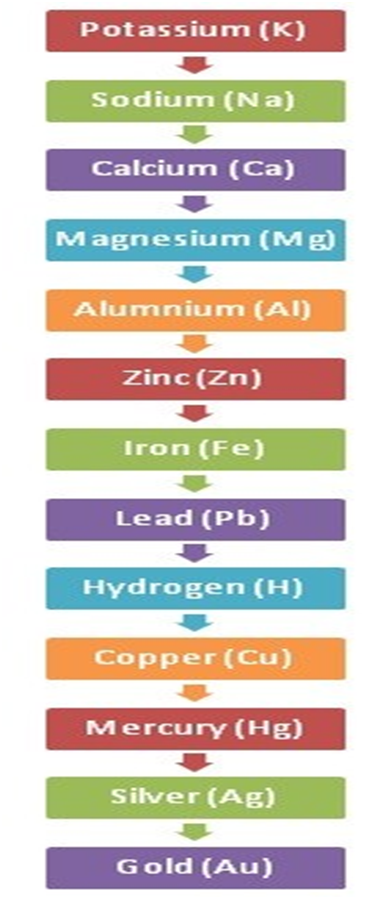
The reaction of Different Metals and Non-metals with Acids
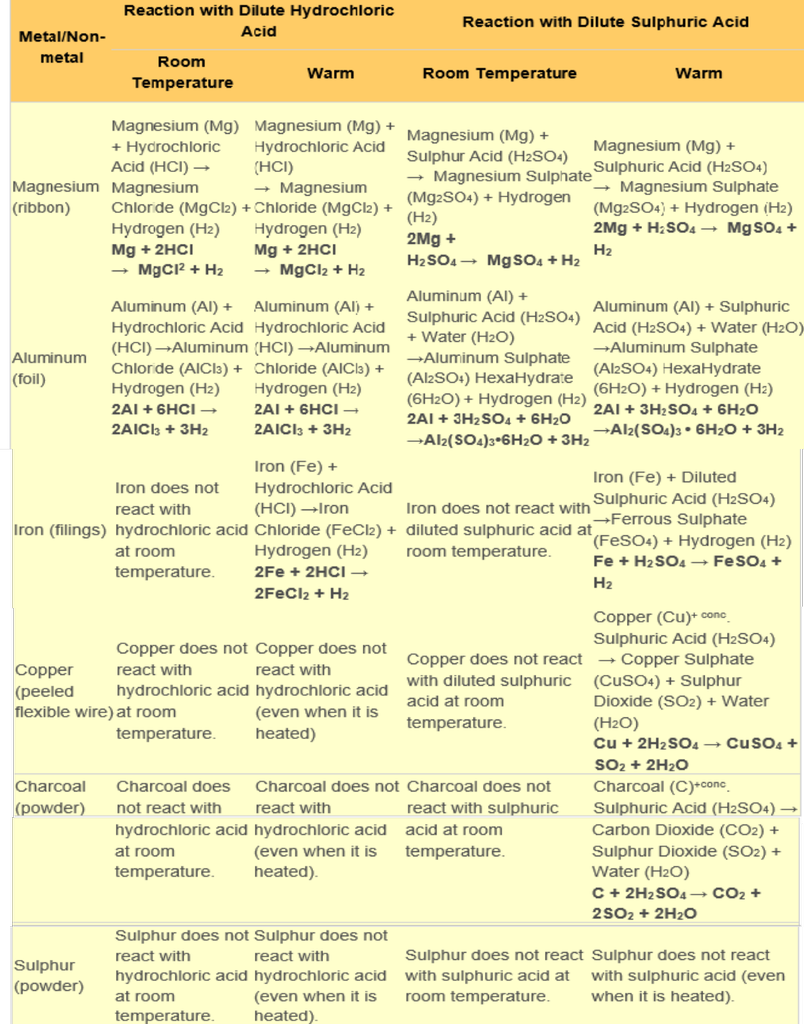
Note:
- When metals react with acids, they produce hydrogen gas with produces a 'pop' sound when it burns.
- Iron reacts with hydrochloric acid and sulphuric acid on heating.
- Copper does not react with hydrochloric acid (even when it is heated) but reacts with sulphuric acid on heating.

 PathSet Publications
PathSet Publications
