- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
Do Liquids Conduct Electricity?
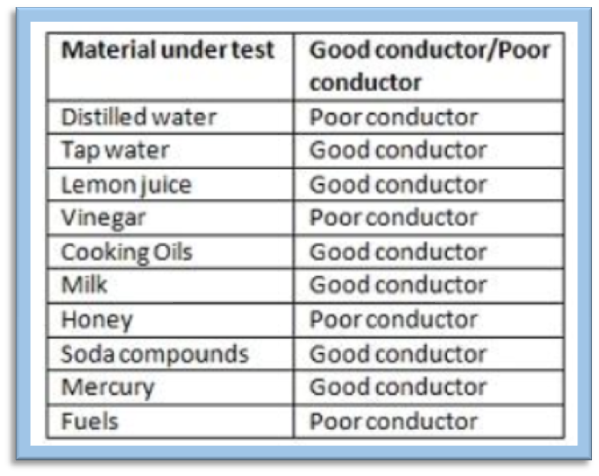
Under certain conditions, most of the materials can conduct electricity. Hence, it is preferable to classify materials as good conductors and poor conductors instead of classifying them as conductors and insulators.
• Materials that allow an electric current to pass through them, are good conductors of electricity.
• Materials that do not allow an electric current to pass through them easily, are poor conductors of electricity.
The conductivity of Liquids:-
Liquids that allow the passage of electricity through them are known as conducting liquids. Such liquids are known as electrolytes.
Liquid as a Conductor of Electricity:-
• The liquids that conduct electricity are solutions of acids, bases and salts.
• Distilled water is free from salts. Hence, it is a poor conductor.
• When salt is dissolved in distilled water, we obtain a salt solution. This solution is a conductor of electricity.
• Tap water is a good conductor of electricity as small amounts of minerals and salts are naturally present in it.
• Not all liquids can conduct electricity. However, some of them can be regarded as good conductors of electricity while others as poor conductors of electricity.
• Water containing salts and minerals dissolved in it always conducts electricity.
• Distilled water that does not contain any salts cannot conduct electricity.
• Any solution of acids or bases can also conduct electricity.
• Different substances when mixed in water and electricity is passed through them can break apart and form positive and negative particles or ions in the water.
• These ions can pass the electric current through them.
• The more is the number of ions in a liquid the better conductor it is of electricity.
• That is why distilled water is a poor conductor of electricity but salt water is a good conductor of electricity.
• However, many compounds do not form any ions on mixing them with water and therefore they are poor conductors of electricity such as sugar water, oil and alcohol.
Why LED bulbs are more suitable for testing the electrical conductivity of liquids?
• The electric current often causes a heating effect due to which the filament of the bulb gets heated up and glows.
• However, some liquids are capable of conducting electricity but they are weak conductors of electricity. Hence current passes through them but it is not that strong enough to heat up the filament. As a result, the filament would not light up in the case of such liquids.
• However, the LED bulbs can detect the flow of even a small amount of electric current as well. Hence, LED bulbs are suitable for testing the electrical conductivity of liquids.
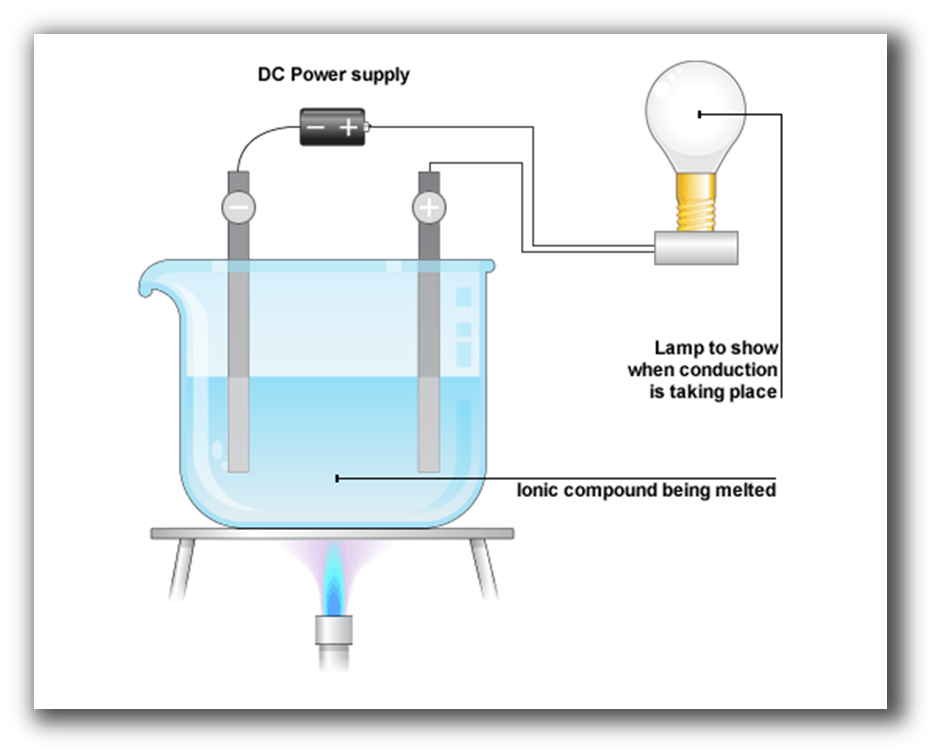
What is Electrolysis?
The effect in which components of a compound get split due to passing an electric current through it is called electrolysis.
What is an Electrode?
An electrode is a conductor of electricity that can carry electric current into non-metals and other poor conductors of electricity.
What is an Electrolyte?
A solution that breaks into its ions on passing electricity through it is called an electrolyte. Electrolytes are used in the process of electroplating.
What are an Anode and Cathode?
The positively charged electrode is called an anode and the negatively charged electrode is called a cathode.
What are Anions and Cations?
An anion is a negatively charged ion and a cation is a positively charged ion.
Chemical Effects of Electric Current
Effects of an Electric Current
- Heating effect: electric current causes heating of the electrical equipment. For example, the filament of a bulb gets heated up due to electric current and therefore glows.
- Mechanical effect: an electric current can lead to the generation of mechanical energy in appliances. For example, fans and motors work due to this effect.
- Magnetic effect: an electric current can give rise to the magnetic field of a substance.
- Chemical effect: an electric current can lead to the production of chemical energy or chemical reactions.
Chemical Effects of Electric Current
We know that when an electric current passes through a solution it ionizes and breaks down into ions. This is because of chemical reactions that take place when an electric current passes through a solution. Depending on the nature of the solution and the electrodes used, the following effects can be observed in the solution:
- Metallic deposits on the electrodes
- Change in the colour of the solution
- A release of gas or production of bubbles in the solution

 PathSet Publications
PathSet Publications
