Physical Properties of Metals and Non-metals
- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
Physical Properties of Metals and Non-Metals
Elements can be classified into the following two groups depending on their physical and chemical properties:
- Metals
- Non-metals
Metals
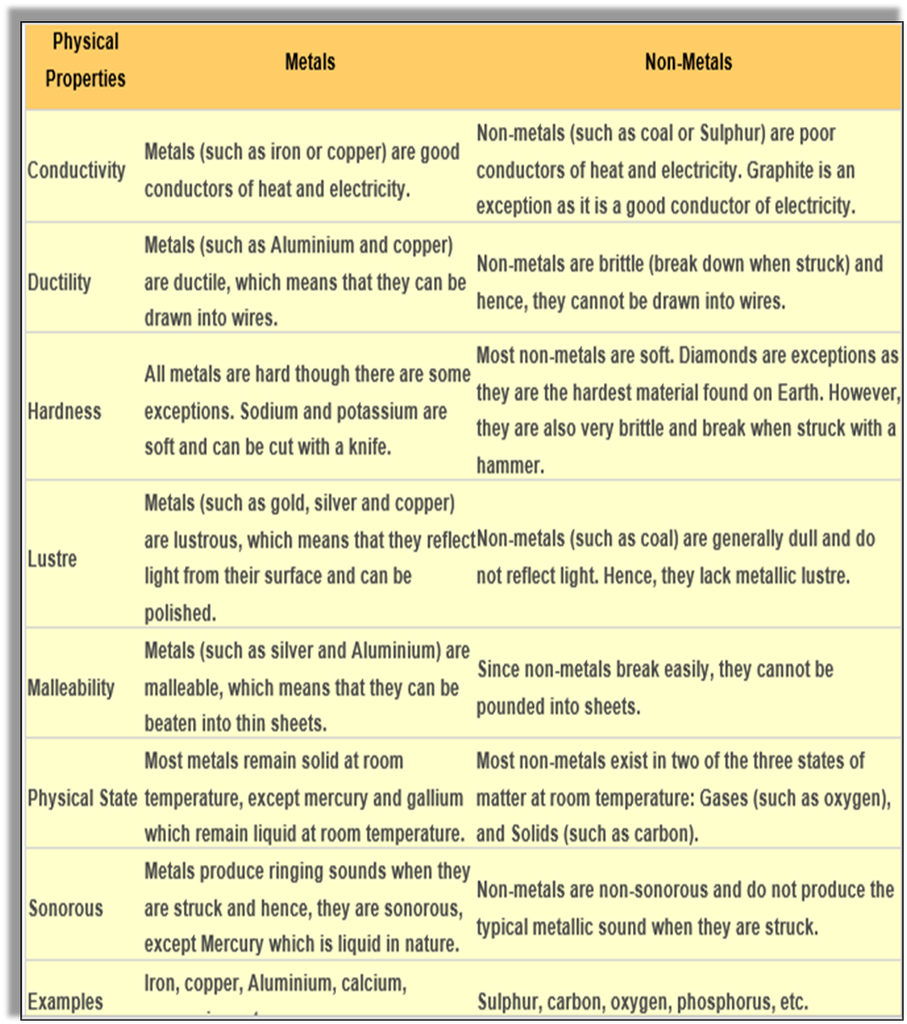
Metals are opaque and lustrous elements that are good conductors of heat and electricity.
Example: Iron, copper, zinc.
- The elements which are hard, shiny can be beaten into sheets, drawn into wires and are good conductors of heat and electricity are generally metals. For example iron, copper, gold, etc.
- In nature, most metals occur in the combined state as minerals and they are reactive.
- Only a few unreactive metals like gold, silver, platinum are found as free metals in the earth’s crust.
- Minerals from which metals can be profitably extracted are called ores. For example, calcium occurs in limestone (calcium carbonate) or iron in the ore Haematite.
Physical properties of metals:
Malleable – ability to be beaten into sheets. The property by virtue of which metal can be beaten into sheets is called malleability. Eg- We use Aluminum foil to pack food. Aluminum and silver foil.
Ductile – ability to be drawn into wires. The property by which metals can be drawn into wires is called ductility. Metals like copper, silver and Aluminum can be drawn into wires.
Lustrous – ability to shine. Metals such as gold, silver and copper all have lustre, that is they have an ability to shine and reflect light. Therefore, they are lustrous.
Sonorous – makes a ringing sound when strikes with a hard surface, e.g. bells.
Hard in nature -e.g. iron, lead
Exception – (Sodium and potassium are soft metals and can be cut with a knife).
Good conductors of heat and electricity. They are good conductors of heat and electricity. Copper is the best conductor of electricity followed by gold and Aluminum.
Mostly solids Exception – (Mercury is the only liquid metal).
Mercury - is the only metal that is found in the liquid state at room temperature.
Metals like Sodium and Potassium are soft and can be cut with a knife.
High melting and boiling point -With the exceptions of sodium, potassium and mercury most of the metals have high melting and boiling point.
High Densities -Metals have high densities.
High tensile strength - Most metals have high tensile strength.
Non- Metals
The elements that do not have the properties of metals are known as non-metals. Non-metals are widely present in the earth’s crust and atmosphere. All oceans and water bodies have an abundance of these elements. Examples: Carbon, hydrogen, and oxygen.
- The elements which are brittle, dull cannot be beaten into sheets or drawn into wires and are poor conductors of heat and electricity are generally non-metals.
- For example, oxygen and nitrogen occur in a free state in the air and in the combined state in earth’s crust. Sulfur occurs both in the free and the combined state in the earth’s crust.
- The noble gases, helium, neon, argon, krypton, xenon occur only in Free State.
Physical properties of Non-Metals:
▪ Non-malleable – they are not malleable cannot be made into sheets e.g. Sulphur breaks into powdery mass when beaten with a hammer.
▪ Non-ductile – Non-metals are brittle that is they are not ductile. Hence, cannot be made into wires.
▪ Non-lustrous/dull - Non-metals do not have lustre except iodine and graphite. e.g. coal.
▪ Non-sonorous – cannot make a ringing sound when struck with any object.
▪ Soft, break easily, e.g. Sulphur, Pencil lead.
▪ Poor conductors of heat and electricity, e.g. phosphorus
▪ Exception – (Graphite is a good conductor of heat and electricity).
▪ Non-metals are gases or solid at room temperature, except bromine which is liquid at room temperature. It can be solid (Sulphur), liquid (bromine), or gaseous (oxygen). They usually have low densities. Non-metals have low melting points and boiling points.
Physical Properties of Metals and Non-metals
Note: Copper Vessels also acquire a dull green coating (made up of copper hydroxide and copper carbonate) when they are exposed to moist air. It is called Verdigris.
Chemical Properties of Metals and Non-metals
- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
Chemical Properties of Metals and Non-metals
Reaction of Metals with Oxygen:
When metals react with oxygen, they form metal oxide.
Metal + O2 → Metal Oxide
For Example:
1. Copper + Oxygen → Copper Oxide (black)
2Cu + O2 → 2CuO
2. Aluminium + Oxygen → Aluminium Oxide
4Al + 3O2 → 2Al2O3
3. Magnesium + Oxygen → Magnesium Oxide
2Mg + O2 → 2MgO
The reactivity of metals with oxygen differs. For Example:
- Sodium and Potassium react so vigorously with air that they catch fire when they are kept in open. Hence, they are immersed in kerosene to store them.
- The surfaces of Magnesium, Aluminium, Zinc and Lead are covered with a thin layer of oxide to make sure that they do not get oxidized anymore.
- While iron does not burn when heated, iron filings burn vigorously.
- The copper surface is coated with a thin layer of black copper oxide to prevent the process of further oxidation from taking place.
- Gold and silver do not react with Oxygen.
Amphoteric Oxides: Some metal oxides react both with acids and bases to produce salts and water. Such metal oxides are called amphoteric oxides. For example:
Aluminum Oxide + Hydrochloric Acid → Aluminium Chloride + Water
Al2O3 + 6HCl → 2AlCl3 + H2O
Aluminum Oxide + Sodium Hydroxide → Sodium Aluminate + Water
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Reaction of Metals with Water
Metal + (cold) Water → Metal hydroxide + Hydrogen
Metal + Stream → Metal oxide + Hydrogen
For Example:
Sodium + Water → Sodium Hydroxide + Hydrogen + Heat
2Na + 2H2O → 2NaOH + H2 + Heat
Calcium + Water → Calcium Hydroxide + Hydrogen
Ca + 2H2O → Ca(OH)2 + H2
Magnesium + Water → Magnesium Hydroxide + Hydrogen
Mg + 2H2O → Mg(OH)2 + H2
Aluminium + Water → Aluminium Oxide + Hydrogen
2Al + 3H2O → Al2O3 + 3H2
Iron + Water → Iron Oxide + Hydrogen
3Fe + 4H2O → Fe3O4 + H2
The reactivity of metals with water differs. For Example:
- Sodium, Potassium and Calcium react with cold water.
- Magnesium reacts slowly with hot water to form slightly soluble magnesium hydroxide.
- Aluminum, Zinc and Iron react with steam.
- Lead, Copper, Silver and Gold do not react with water.
Note: Calcium and magnesium float on water as bubbles of hydrogen stick to their surface.
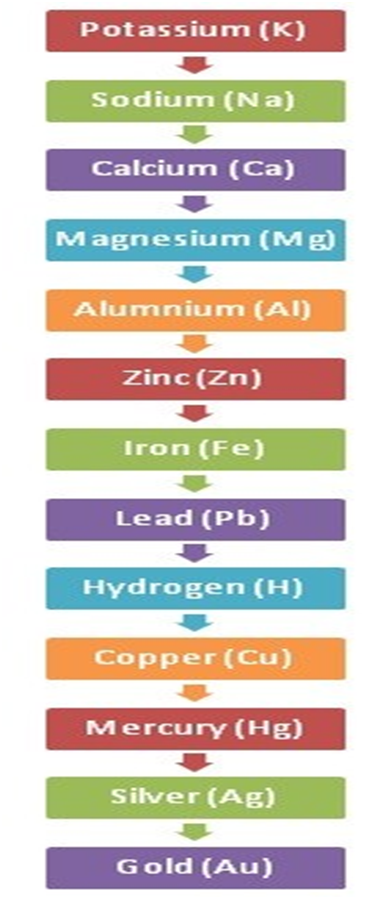
The reaction of Metals with Solutions of Other Metal Salts
Metal A + Salt Solution of Metal B → Salt Solution of Metal A + Metal B
More reactive metals replace less reactive metals.
Less reactive metals cannot replace more reactive metals.
For Example:
Iron + Copper Sulphate → Iron Sulphate + Copper
Fe + CuSO4 → FeSO4 + Cu
The reactivity series is given below:
The reaction of Different Metals and Non-metals with Acids
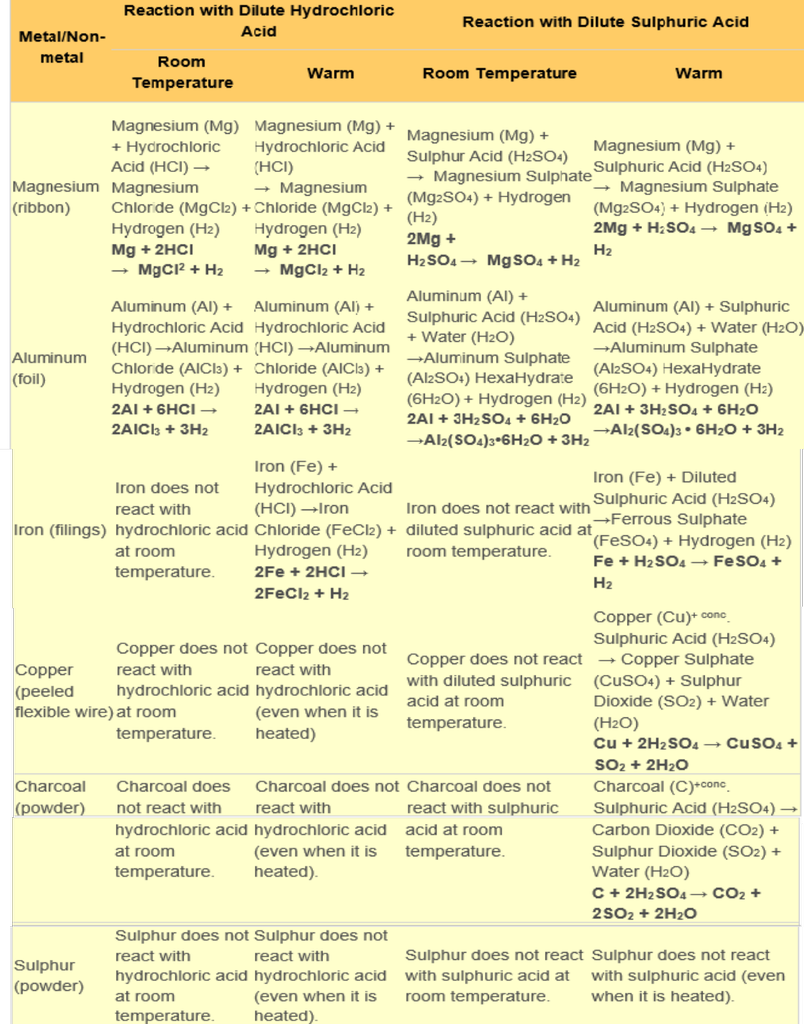
Note:
- When metals react with acids, they produce hydrogen gas with produces a 'pop' sound when it burns.
- Iron reacts with hydrochloric acid and sulphuric acid on heating.
- Copper does not react with hydrochloric acid (even when it is heated) but reacts with sulphuric acid on heating.
Uses of Metals and Non-metals
- Books Name
- Class-8 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 8
- Subject
- Science
Uses of Metals and Non-metals
As discussed above, metals are hard, malleable, ductile, and sonorous and are hence, can be used for:
- Making machinery
- Making automobiles, trains, and airplanes
- Making cooking utensils and water boilers
- Making industrial gadgets and satellites etc.
Non-metals also have several uses, such as:
- Essential for life (such as oxygen)
- Used as fertilizers (such as nitrogen and phosphorus)
- Used to purify water (such as chlorine)
- Applied on wounds as an antiseptic (such as purple-colored iodine solution)
- Used in crackers (such as Sulphur)
- Oxygen –required for breathing.
- CO₂–needed for photosynthesis.
- Nitrogen, Sulphur, Phosphorus –present in DNA, RNA; also used to make fertilizers for plants.
- Carbon and hydrogen are the main elements in all biomolecules.

 PathSet Publications
PathSet Publications
