- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
CRYSTALLIZATION
Closely observe common salt and sugar. we will notice that all common salt and sugar particles are of uniform shape and size, i.e., almost cubical. Such uniform structures are called crystals.
In a crystal, atoms are arranged in a regular pattern. The crystals of common salt, sugar, alum, etc. are obtained from the solutions of these substances in water by a process called crystallization.
During crystallization, a solid is first dissolved in water. Then the water in the solution formed is allowed to evaporate. By this method, large crystals of pure substances can be obtained.
Seawater contains salt. Salt is obtained by collecting seawater in shallow ponds. The water gets evaporated under the heat of the sun and solid salt is left behind.

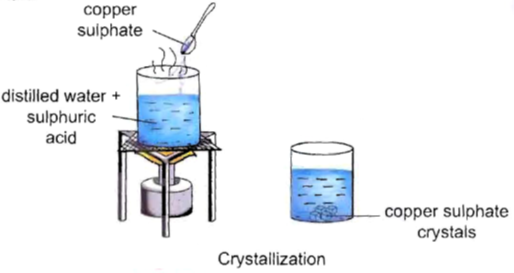
Activity–5
To observe the process of crystallization.
Procedure:
• Take 50 mL of distilled water in a 100 mL beaker.
• Add 2-3 drops of dilute sulphuric acid to it.
• Heat it over a burner.
• When it starts boiling, add a small amount of copper sulphate powder to it.
• Stir it continuously to dissolve.
• Continue adding copper sulphate powder till no more powder can be dissolved.
• Carefully filter the hot solution.
• Leave it undisturbed overnight.
Observation : We will observe clean blue crystals of copper sulphate at the bottom of the beaker.
Inference : Crystals of copper sulphate are formed by the process of crystallization.
Key Words
1. Chemical change : These are the changes in which chemical properties of substances are changed and new substances are formed.
2. Chemical reaction : The process involving chemical changes are accompanied with chemical reaction.
3. Crystallisation : It is the process of getting crystals of pure substance from their solutions.
4. Galvanisation : The process of depositing a layer of zinc on iron is called galvanisation.
5. Physical change : A change in only physical properties of a substance is called physical change.
6. Rusting : If a piece of iron is left open for some time, it acquires a film of brownish substance. This substance is called rust and the process is called rusting.

 Grow Career Publication
Grow Career Publication
 Param Publication
Param Publication
