Physical Changes
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
Physical Change
The properties such as size, shape colour and state of a substance are called its physical properties.
When a substance undergoes a change in its physical properties, that change is said to be physical change. During a physical change, no new substance is formed. Physical changes are generally reversible changes.
Remember
• Reversible change:
A change in which we can get back the initial substance by reversing the action is an reversible change.
Activity–1
To observe a physical change with the help the of ice cubes. 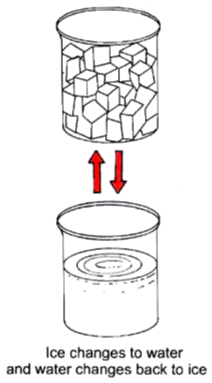
Procedure :
• Take a few ice cubes in a beaker.
• Keep them in the open for 4–5 minutes.
Now ,we will observe that ice (solid) changes into water (liquid). There is a change in the state of ice from soild to liquid.
• Now pour this water back into the ice tray and keep it in the freezer for 30 minutes.
Now, we will get back the ice. Therefore, it is a physical change.
Activity–2
To observe a physical change with the help of a sugar solution.
Procedure : 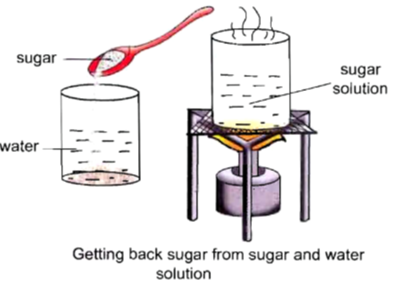
• Take 100 mL of water in a beaker.
• Dissolve a spoonful of sugar into it.
• Now, we will get a solution of sugar and water.
• Now, heat this solution over a burner for sometime.
• Now, we will observe that slowly the water evaporates and sugar is left at the solution.
• We will be able to get back sugar. Therefore, it is a physical change.
Characteristics of physical change
(i) No new or different product is formed : The composition of molecules of the substance remains unaltered.
Example : Ice melts to form water. In this example only the appearance (state) of matter has changed from solid to liquid. However, the composition of the molecules of ice or water remains same, i.e., for every 1 g of hydrogen, 8 g of oxygen is required . Thus, only a physical change has occurred.
(ii) The change is temporary and is usually reversible : It means the change can be reversed by altering the causes which produce the change.
Example : The water formed from ice can be changed back to ice by placing it in a freezing mixture (a mixture of ice and common salt).
(iii) There is no change in the weight of substance : During a physical change it is only the energy which is added or removed. No matter is added during a physical change. Similarly, no matter is removed during a physical change. Therefore, mass of the substance remains the same.
Some Examples Involving Physical Changes :
Some Common Examples of Physical Changes
• Formation of dew.
• Evaporation of water.
• Crystallisation of sugar from its solution.
• Ringing of an electric bell.
• Breaking of a glass pane.
• Freezing of ice cream.
• A rock rolling down a hill.
• Bending of a glass tube by heating.
• Melting of wax.
• Sublimation of camphor.
Chemical Change
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
Chemical Change
A change which alters the specific properties of a material by bringing about a change in its molecular composition, is called a chemical change.On the other hand we can say that when two or more substances reacts in such a way that there is formation of one or more new substances, the change is called a chemical change or a chemical reaction.
During a chemical change, reactants undergo changes to form products. Chemical changes, generally, are irreversible changes.
Advance Learning
• Chemical equations :
All chemical changes are accompanied by chemical reactions. These reactions can be described in sentence form, but the description would be quite long. Chemical equations have been framed to describe the chemical reactions.
A chemical equation links together the substances which react with the new substances that are formed.
• Reactants :
The substances which take part in bringing about chemical change are called reactants.
• Products :
The substances which are produced as a result of chemical change are called products.
![]()
• These reactions involve breaking and making of chemical bonds.
Remember
• Irreversible change:
A change in which we cannot get back the initial substance by reversing the action is an irreversible change.
Activity–3
To observe the formation of a new substance on heating a magnesium ribbon and then adding water to it.
Procedure: 
• Take a small piece of a magnesium ribbon.
• Clean its tip by rubbing it with a sandpaper.
• Hold it with a pair of tongs over the flame of a burner.
Now, we will observe that it burns with a brilliant white light. It leaves behind a powdery ash after burning. The ash obtained is not the same as the magnesium ribbon. Magnesium has lost its properties and a new substance, magnesium oxide (MgO) is formed.
• Collect the ash in a beaker and mix it with a small amount of water.
• Stir it properly.
• With the help of a dropper, put a drop of this solution on red and blue litmus papers to test its chemical nature.
Now, we will observe that red litmus paper turns blue and blue litmus paper remains as it is. This shows that the solution obtained is basic in nature.
Inference : Magnesium oxide, on dissolving in water, forms magnesium hydroxide which is a new substance. Thus, it is a chemical change.
This change is represented as:
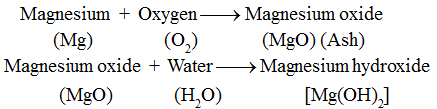
Activity–4
To observe the formation of a new substance on putting an iron nail in copper sulphate solution.
Procedure : 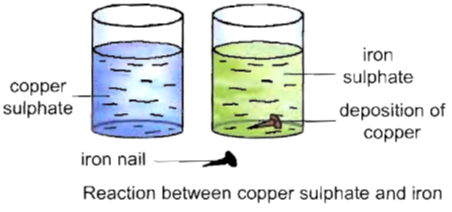
• Take 100 mL water in a 250 mL beaker.
• Dissolve a spoonful of copper sulphate (blue vitriol or neela thotha) in it.
• Add a few drops of dilute sulphuric acid to it.
Now, we will get a blue-coloured solution.
• Divide this solution into two equal parts.
• To the second part, drop an iron nail and leave it for 30 minutes.
• Compare the colour of this solution with the second part of the solution .
Now, we will observe that the blue colour of the solution changes to green colour. Also a brown-coloured layer get deposited on the iron nail.
Inference :
Copper sulphate solution is blue in colour. It changes to green colour due to the formation of a new substance, i.e., iron sulphate. The brown deposit on the iron nail is of copper, another new substance. Hence, this is a chemical change.
This change can be represented as :
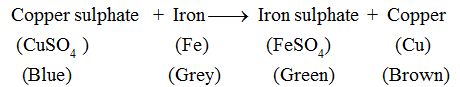
Advance Learning
• Displacement reaction
Iron is a more reactive metal than copper. Iron replaces copper from copper sulphate solution to form iron sulphate. Such reactions, in which a more reactive metal replaces a less reactive metal from its salt solution is called displacement reaction.
• When we leave cut slices of apple, brinjal, potato, etc. they acquire a brown-coloured layer. This change of colour is due to the formation of a new substance on reaction with atmospheric air.

Rusting of Iron
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
RUSTING OF IRON
When iron objects are left exposed to moist air (oxygen and water both), a substance with a brown flaky layer is observed on their surfaces. This brown flaky layer is hydrated iron oxide. It is called rust.
Rust falls off the surface, exposing the iron surface beneath. Rusting of iron is a slow change that destroys the whole iron object.
Iron is an important metal. It is used in making bridges, cars, ships, trucks, gates, benches and various other useful articles. Every year, a lot of monetary loss occurs due to damage of iron articles by rusting.
The process of rusting of iron is represented as:
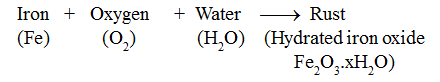
Remember
• Rusting of iron takes place in the presence of both oxygen and water (or water vapour). If anyone of these is not present, rusting will not occur.
Advance Learning
• Rusting of iron becomes faster if the content of moisture in the air increases.
• Rusting is faster in salty water.
Prevention of Rusting of Iron
Rusting of iron can be prevented in many ways.
(i) By avoiding direct contact with air and moisture :
It is done by using the following methods :
• Applying grease or oil on the exposed parts of iron articles.
• Painting the surface of iron articles.
• Galvanizing the surface of iron articles. Galvanization is a process in which a layer of metals like chromium or zinc is deposited on the surface of iron articles electrolytically, i.e., by passing electric current.
• Electroplating the surface of iron articles with metals, which are not attacked by atmospheric moisture. The shining parts of bicycles are given a coating of chromium (chrome plating) to protect them from rusting.
(ii) By alloying : When mixed with certain corrosion resistant metals or some non-metals, iron forms alloys which are resistant to rusting. Stainless steel, an alloy of iron, nickel and chromium does not rust.
Advance Learning
• Electroplating
It is the deposition of a metallic coating (say gold) by passing electric current through a solution containing dissolved metal ions and the metal object to be electroplated.
This is the process by which wrist watches, jewellery and other items are plated with gold.
Crystallization.
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
CRYSTALLIZATION
Closely observe common salt and sugar. we will notice that all common salt and sugar particles are of uniform shape and size, i.e., almost cubical. Such uniform structures are called crystals.
In a crystal, atoms are arranged in a regular pattern. The crystals of common salt, sugar, alum, etc. are obtained from the solutions of these substances in water by a process called crystallization.
During crystallization, a solid is first dissolved in water. Then the water in the solution formed is allowed to evaporate. By this method, large crystals of pure substances can be obtained.
Seawater contains salt. Salt is obtained by collecting seawater in shallow ponds. The water gets evaporated under the heat of the sun and solid salt is left behind.

Activity–5
To observe the process of crystallization.
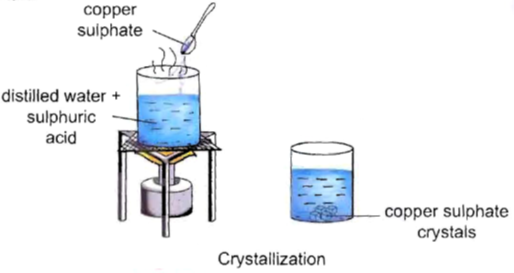
Procedure:
• Take 50 mL of distilled water in a 100 mL beaker.
• Add 2-3 drops of dilute sulphuric acid to it.
• Heat it over a burner.
• When it starts boiling, add a small amount of copper sulphate powder to it.
• Stir it continuously to dissolve.
• Continue adding copper sulphate powder till no more powder can be dissolved.
• Carefully filter the hot solution.
• Leave it undisturbed overnight.
Observation : We will observe clean blue crystals of copper sulphate at the bottom of the beaker.
Inference : Crystals of copper sulphate are formed by the process of crystallization.
Key Words
1. Chemical change : These are the changes in which chemical properties of substances are changed and new substances are formed.
2. Chemical reaction : The process involving chemical changes are accompanied with chemical reaction.
3. Crystallisation : It is the process of getting crystals of pure substance from their solutions.
4. Galvanisation : The process of depositing a layer of zinc on iron is called galvanisation.
5. Physical change : A change in only physical properties of a substance is called physical change.
6. Rusting : If a piece of iron is left open for some time, it acquires a film of brownish substance. This substance is called rust and the process is called rusting.
Chemical Change (Part -2)
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
Some Common Examples of Chemical Changes
• Burning of wood or charcoal • Burning of candle • Digestion of food • Curdling of milk
• Formation of biogas (Gobar gas) • Burning of petrol or diesel
• Drying of paint • Rusting of iron
• Ripening of fruit •Clotting of blood • Fading of the colour of a dyed cloth
• Baking of cake • Photosynthesis • Formation of wine • Butter turning rancid
• Electrolysis of water into hydrogen and oxygen • Formation of water from hydrogen and oxygen
Characteristics of chemical change
In addition to the formation of new substances, a chemical change may be accompanied by one or more of the following six changes :
(i) Evolution of gas
For example:
• When zinc granules are added to dilute sulphuric acid, hydrogen gas is evolved. If a burning matchstick is brought near the mouth of a test tube, it burns with a pop sound.
![]()
(ii) Change of colour
For example :
• When solid lead nitrate is heated, reddish-brown nitrogen dioxide gas is evolved. Also, a yellow-coloured lead monoxide is formed.
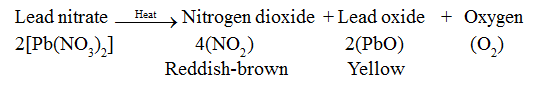
(iii) Formation of precipitate
Precipitate is a solid substance that deposits from a solution .
For example:
• When hydrogen sulphide gas is passed through blue coloured copper sulphate solution, black precipitate of copper sulphide is formed . .
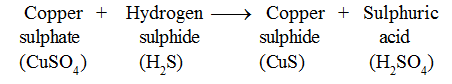
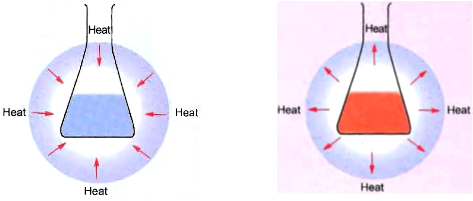
(iv) Absorption or evolution of heat, light or any other radiation
For example :
• When carbon and sulphur are heated, i.e., heat energy is absorbed, then carbon sulphide is formed.
![]()
• When water is added to quicklime, heat energy is evolved.
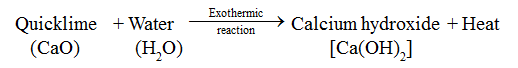
Advance Learning
• Endothermic Reaction
The reaction in which heat energy is absorbed is called endothermic reaction.
• Exothermic Reaction
The reaction in which heat energy is evolved is called exothermic reaction.
(v) Sound may be produced
For example :
• when baking soda is added to vinegar, carbon dioxide gas is produced with a hissing sound.
![]()
(vi) Change of smelI may occur or a new smell may be given off
For example :
• When cooked food containing oils and fats is kept in the open (not refrigerated) for long, it gets spoiled and gives a foul smell.
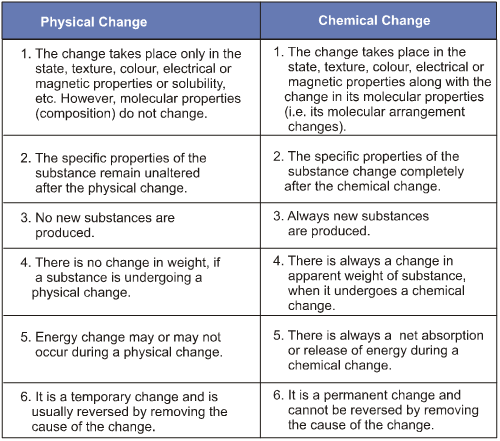
Difference Between Physical and Chemical Changes
Corrosion
It is a gradual deterioration of metals on interaction with their environment as a result of chemical changes between them. Almost every metal is susceptible to degradation. Rusting of iron and tarnishing of silver are common examples of corrosion.
Corrosion decreases their intended usage period. The strength and appearance are also degraded.

 Grow Career Publication
Grow Career Publication
 Param Publication
Param Publication
