Physical Changes
Chapter 6: Physical and Chemical Changes
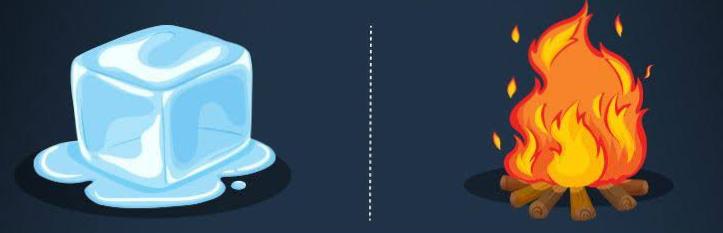
Matter is capable of undergoing changes, which are classified as either physical or chemical. Physical changes in matter are reversible: An ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. Chemical changes, on the other hand, are not reversible: A log burned in a fire turns to ashes, but the ashes cannot be changed back into a log
Physical changes
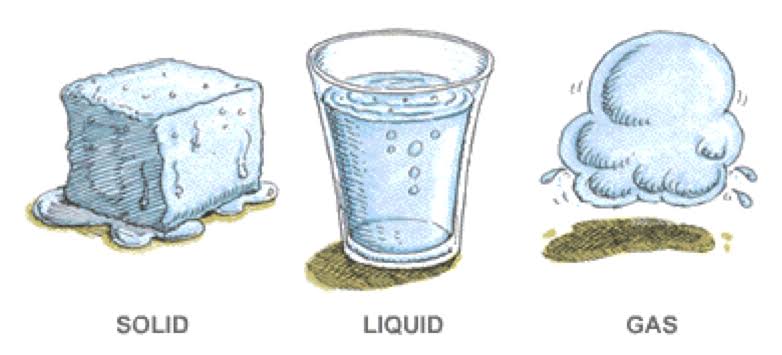
In a physical change, the material involved in the change is structurally the same before and after the change. Types of some physical changes are texture, shape, temperature, and a change in the state of matter. A change in the texture of a substance is a change in the way it feels. For instance, a block of wood may feel rough when you run your finger across it but rubbing the wood with sandpaper smooths the surface so it no longer feels rough. The wood itself has not changed during sanding to become a new material, only the texture of the surface changed. A piece of metal may be heated in a fire until it glows, but the metal is the same material before heating and after cooling. Similarly, when a material changes phase, it only changes physically; the substance is still the same.
Chemical Change
Chemical change

A chemical change occurs when the composition of a substance is changed, which requires the breaking and forming of chemical bonds during a chemical reaction. This results in the rearranging of atoms in substances to form the products of a chemical reaction, which are brand new molecules that cannot be easily reverted back to their original state.
Rusting of Iron
Rusting of iron

Rusting is an oxidation reaction. The iron reacts with water and oxygen to form hydrated iron(III) oxide, which we see as rust. Iron and steel rust when they come into contact with water and oxygen – both are needed for rusting to occur.
Crystallization.
Crystallization
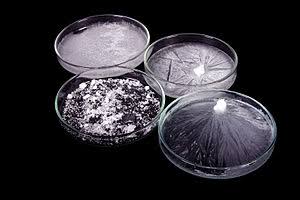
Crystallization or crystallisation is the process by which a solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some of the ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas.

 Param Publication
Param Publication
 Grow Career Publication
Grow Career Publication
