Hot and Cold
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
INTRODUCTION :
Heat is a form of energy. We know that energy means capacity to do work. So we can say, heat, being a form of energy, can do work. For example, when pressure cooker is heated, steam is formed within the cooker and this steam can lift the lid of the pressure cooker. In this chapter we shall study about the meaning of hot and cold and how things are heated or cooled.
TEMPERATURE - DEGREE OF HOTNESS ORCOLDNESS :
When we touch an ice cube, we say it is cold. Similarly when we touch a cup of tea, we say it is hot. Here we compare the hotness or coldness of an object with that of our hand. But sensing the hotness or coldness by touching is not always true. “The degree of hotness or coldness of a substance is called its temperature.” When two objects at different temperatures are kept in contact, heat flows from hotter object (high temperature) to colder object (low temperature). So direction of heat flow is governed by the temperatures of the objects in contact. It should be kept in mind that exchange of heat continues so long as the temperatures of the two objects remains unequal. Heat flow stops as soon as temperatures become equal which is known as thermal equilibrium.
Differences Between Heat and Temperature :
Measuring Temperature
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
Measurement of temperature :
Temperature is measured by an instrument called thermometer.
A simple thermometer has a long, narrow, uniform glass tube at one end of which there is a glass bulb filled with mercury.
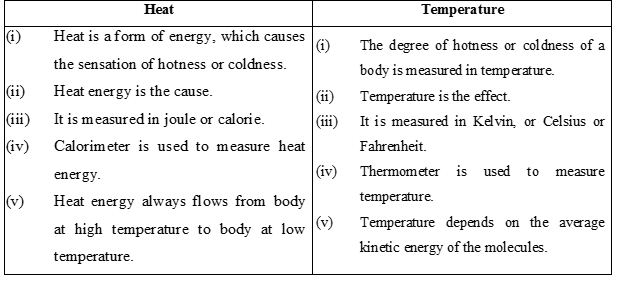
Along the length of the glass tube, there are graduations (Scales) made on the basis of two standard temperatures. These are called lower fixed point and upper fixed point.
Lower fixed point is the temperature at which ice melts and upper fixed point is the temperature at which water boils.
There are two commonly used temperature scales
(i) Celsius Scale (ii) Fahrenheit scale (iii) Kelvin Scale
(a) Celsius or Centigrade Scale :
As the name suggests, this scale has 100 divisions between the upper and lower standard points. This scale was introduced by a Swedish astronomer Celsius and is known after his name. Each division on this scale is called one degree centigrade or one degree Celsius and is written as 0C. More sensitive thermometers have 200 divisions between standard points and each division is equal to ½ 0C. Sometimes these thermometers are called half 0C thermometers.
(b) Fahrenheit Scale :
This scale was introduced by Fahrenheit. On this scale 320 F represents the melting point of ice and
2120 F the steam point. Zero is marked 320 F below the ice point. The length in between the standard points is divided into 180 equal parts. Each division on this scale is called 10 F. This scale is widely used for meteorological and clinical purposes.
Relation between Celsius and Fahrenheit scales :
![]()
Where C and F are the corresponding temperatures shown on the scales of thermometers in Celsius and Fahrenheit units respectively.
![]()
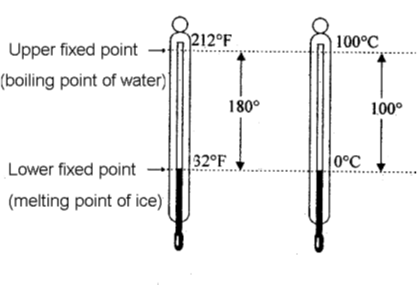
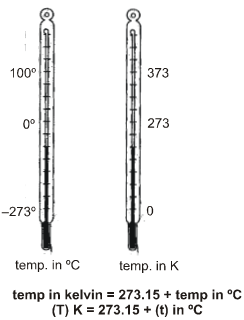
(c) Kelvin Scale :
The scale of measurement of temperature, in which lowest temperature is zero Kelvin (–2730 C) is called
Kelvin scale. This is also called S.I. scale of temperature.
• Characteristics of Kelvin scale :
(i) There cannot be any temperature below zero Kelvin.
(ii) The temperature is expressed in (K), but no degree symbol is attached to it.
(iii) Rise in temperature in kelvin = Rise in temperature in degree Celsius.
Note : Conversion of temperature from Kelvin scale to Celsius scale is K = (C + 273)
• Relation between different temperature scales :
![]()
Laboratory Thermometer
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
Type of Thermometer :
(a) Clinical thermometer :
It is a specially adapted Fahrenheit thermometer used by doctors to record the temperature of human body.
The markings are from 950 F to 1100 F, because the temperature of human body does not fall below 950 F or rise above 1100 F, as in either case death occurs. Some clinical thermometers have centigrade scale, marked between 350 C to 430 C, the normal temperature of human body being 370 C. Before using the thermometer it should be washed with water and then jerked so that the mercury flows back into the bulb.
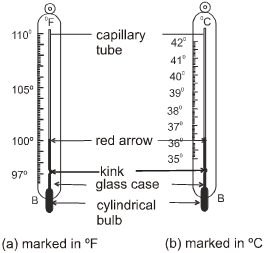
(b) Laboratory Thermometer :
Different types of thermometers are used for different purposes. The maximum and minimum temperatures of the previous day, reported in weather reports, are measured by a thermometer called the maximum-minimum thermometer.The range of a laboratory thermometer is generally from –10ºC to 110ºC.
In addition to the precautions needed while reading a clinical thermometer, the laboratory thermometer
- Should be kept upright not tilted.
- Bulb should be surrounded from all sides by the substance of which the temperature is to be measured.
- Bulb should not touch the surface of the container.
[Note:- In clinical (Doctor’s) thermometer, Fahrenheit scale is used.
In laboratory thermometer, Celsius scale in used.
SI unit of temperature is kelvin (K)]
Working of Thermometer : 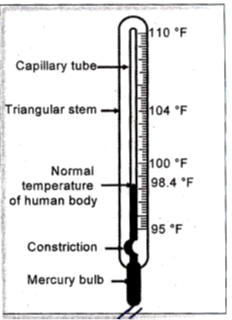
When the Thermometer bulb is in contact with a hot object, mercury column rises up through the capillary tube. The tip of the mercury column in the capillary shows the temperature of the object.
Clinical thermometer is used to measure the temperature of human body.
Clinical thermometers have a kink in the capillary tube. This kink prevents the mercury column to fall down as soon as the thermometer is taken out from the patient’s mouth.
The normal body temperature is 98.6°F (is 37°C). The range of temperature is clinical thermometer is from 94° F to 108°F (35° C to 42° C).
The range of a laboratory thermometer is from –10° C to 110° C
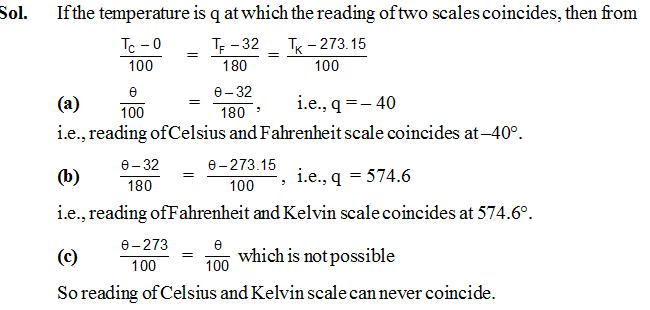
Ex.1 At what temperature, if any, do the following pairs of scales give the same reading :
(a) Celsius and Fahrenheit,
(b) Fahrenheit and Kelvin and
(c) Kelvin and Celsius ?
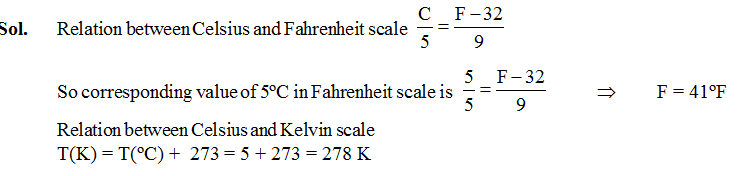
Ex.2 Find the value of 5ºC in ºF and K scale respectively
Transfer of Heat
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
Role of Temperature in Transfer of Heat Energy :
When two bodies of different temperatures are brought in contact with each other, the heat energy always flows from a body at higher temperature to a body at lower temperature, till the temperatures equalise. Thus, it is the temperature of a body which determines the direction of flow of heat energy.
Units of Heat Energy :
Heat energy is measured in calories.
The quantity of heat energy required to raise the temperature of 1 g of pure water through 1 0C (14.50C to 15.50C) is called one calorie.
The calorie is a very small unit of heat energy used for practical purposes. Thus, a bigger unit called kilocalorie is used.
The quantity of heat energy required to raise the temperature of 1 kg of pure water through 1 0C is called one kilocalorie. 1 kilocalorie = 1000 calories.
Kilocalorie is sometimes called Big calorie or Doctor’s calorie or Calorie (with capital C). The energy value of the foods and the fuel is measured in kilocalories.
We know energy is measured in Joules. As heat energy is a form of energy, therefore, it should also be measured in Joules, rather than calories or kilocalories, for strict scientific purpose. However, doctors still continue with kilocalories.
Following are the equivalent of calorie and kilocalorie in joules :
(i) 1 calorie = 4.186 J = 4.2 J (approx)
(ii) 1 Kilocalorie = 4186 J = 4200 J (approx.)
Effects of heat :
(i) Temperature increases when heat is gained by an object.
(ii) Similarly, when an object is cooled (i.e. heat is taken out), its temperature decreases.
(iii) Heat causes expansion (in length, area, volume etc.) of an object
(iv) Heat causes change of state.
Applications of expansion of solids :
1. Fixing of iron rim on wooden cart wheels.
2. Riveting of metal plates.
3. A small gap is left while laying rail tracks.
4. Girders are mounted on the rollers by leaving small space.
5. A sag is left, while laying telephone and electric lines.
6. A small gap is left between the blocks, while laying the concrete roads.
7. Special glasses like borosil or pyrex glasses are used to avoid the breaking of ordinary glass, when hot water is poured.

The bimetallic Strip :
A bimetallic strip is made up of two different metals joined together by riveting. Take a brass and an Invar strip of the same size. Rivet them firmly together as shown in the Fig (a). This is called a bimetallic strip. The principle involved is the unequal expansion and contraction of different materials on heating.
When heated brass expands more and its greater length puts it on the outside of the curve as shown in the Fig.(b).
When it is cooled brass contracts more and its shorter length puts it on the inside of the curve as shown in Fig .(c).
Bimetallic strips are used in fire alarms, thermal switches such as those used in refrigerators, bimetallic thermometer etc.

Specific heat capacity / specific heat :
The amount of heat energy required to raise the temperature of unit mass of a substance through 1°C or 1 K is called specific heat capacity.
Specific Heat of Water :
We have already defined specific heat of a substance. On the same basis, we define specific heat of water as the amount of heat energy required to raise the temperature of unit mass (say, one gram) of water through unit degree (1ºC).
specific heat of water,
s = 1 cal g–1 ºC-1 = 1 cal g–1 K–1
s = 4.2 J g–1 K–1 = 4200 J kg–1 K–1
Specific heat of a substance depends also on the state of the substance i.e. solid, liquid or gas.
Eg.: Specific heat of ice = 0.5 cal g–1 ºC–1;
specific heat of water = 1 cal g–1 ºC–1 and
specific heat of steam = 0.47 cal g–1 ºC–1.
Modes of Transfer of heat :
The materials which allow heat to pass through them easily are called conductor of heat. For example aluminium, iron, copper. The materials which do not allow heat to pass through them are poor conductors of heat called insulators. For example plastic wood.
There are three modes of heat transfer-
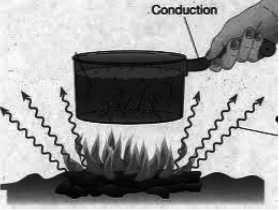
1. Conduction: Conduction is the flow of heat through a substance without the movement of the particles of the substance.
Let us take an example of heating a solid rod by the process of conduction. The molecules in the solid rod are oscillating about their fixed position. As the molecule at one end get heated, they gain kinetic energy and start oscillating vigorously. They collide with the neighbouring molecules and transfer the extra energy to them. These gain kinetic energy and transfer it to their neighbouring molecules. In this way, heat is transmitted from one molecule to the next, down the whole rod, without the molecules actually moving from their positions. This process by which heat travels in solids is called conduction.
Conduction is a process of transfer of heat from the hotter end to the colder end from particle to particle of the medium. Conduction is the process of transmission of heat in solids, in which the molecules of the solid do not move from their position (only oscillate back and forth about their fixed positions) but merely transfer the heat energy in the form of kinetic energy from one molecule to the next.
Thus, medium is required for the transfer of heat by conduction, therefore, conduction is not possible in vacuum. In solids, heat is transferred mainly by the process of conduction.
• Types of conductors :
(i) Good conductors : The substances through which heat energy can easily flow by conduction are called good conductors.
Eg.: Metals in general are good conductors. Amongst the metals, silver is best conductor, next in order are copper, aluminium, gold, etc.
Amongst non-metals graphite is a good conductor.
Metals are good conductor of heat. The high conductivity of metals can be attributed to the presence of a large number of free electrons. These electrons drift away from the source of heat when the metal is heated and in doing so carry the heat energy rapidly through the metal.
(ii) Bad conductors : The substances which do not allow the heat energy to flow through them easily are called poor conductors or bad conductors.
Eg. : Amongst the solid, glass, wood, clay, asbestos, rubber, plastics, wax, etc., are poor conductors.
All liquids except mercury are poor conductors. All gases without any exception are poor conductors.
- Note : Non-metals and organic substances are bad conductors. The low conductivity can be attributed to the lack of a large number of free electrons. It is because most of the heat energy can be transferred only through free electrons and not by the actual vibrational movement of its atoms.
- Practical Applications of Good Conductors :
(i) Copper tubing is used in the automobile radiators, as it readily takes up heat from the hot water coming from the side of engine.
(ii) Cooking vessels are made out of metals, so that they can readily absorb heat energy and transfer it to the food.
(iii) Mercury is used as a thermometric liquid, as it is a good conductor of heat.
(iv) Cooling coils of an air conditioner and the refrigerator are made of copper as they readily conduct heat.
(v) Tip of the soldering rod is made of copper, as it readily conducts away heat to the solder.
- Practical applications of bad conductors : We wear woolen clothes in the winter, because the woolen clothes contains a large amount of the trapped air. Since air is a bad conductor of heat, it does not allow the body heat to flow outward. As our body stops losing heat, we feel warm.
(b) Convection :
Convection is a process of transfer of heat by the actual movement of the medium particles. Liquids and gases are the bad conductors of heat. They are heated mainly by the process of convection. In a solid, the atoms cannot move, leaving their positions. So solids are not heated by convection. A medium is required for the transfer of heat by convection. Heat cannot be transferred by convection in vacuum.
By the process of convection, the transfer of heat is always vertically upwards. The reason is that the medium particles near the source of heat absorb heat from the source and they start moving faster. As a result, the air at this place becomes less dense so it rises up in the medium which is called a convection current. The current continues till the entire liquid acquires the same temperature.
Consequence of convection :
• Land and sea breeze : In the coastal regions, during summer it is noticed that a breeze generally blows from land towards the sea during the night (or early morning) which is called the land breeze.
Land is a better absorber of heat than water. During the day, the land gets hotter, the air above it rises and cooler air from over the sea flows in to take its place. This gives rise to a sea breeze that cools the land.
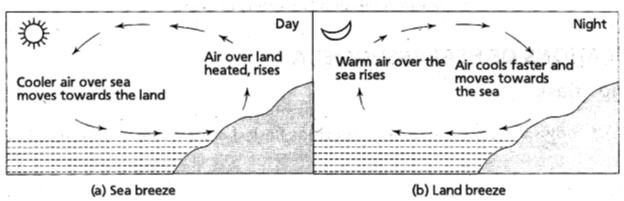
During night, the land radiates the heat it had absorbed during the day and cools down faster than the sea. Above the sea, the air is warmer. It rises and cooler air from the land moves towards the sea to take its place. This gives rise to a land breeze. Thus, we have a sea breeze during day time and a land breeze at night.
(c) Radiation :
Radiation is the process of heat transfer in which heat directly passes from one body to the other body without affecting the medium.
Thus, no medium is required for the heat transfer by the process of radiation. In vacuum, heat transfer takes place only by the process of radiation.
The heat energy transferred by the process of radiation is called the radiant heat or the thermal radiation.
(i) Nature of Radiant Heat : Heat energy is transferred by radiation in the form of electromagnetic waves. These waves can travel even in vacuum. They travel in all directions in straight line with a speed, equal to the speed of light ( = 3 × 108 m s–1). They do not heat the medium through which they pass. They are reflected by a polished and white surface. When radiant heat falls on an object, it is partially absorbed and partially reflected. Dull, black or coloured surfaces are good absorber and good radiators of heat.
(ii) properties of heat radiations :
(A) Heat radiations travel with the speed of light.
(B) Heat radiations can travel through vacuum.
(C) Heat radiations travel in straight lines.
(D) Heat radiations can travel in all directions.
(iii) Applications of heat radiations :
(A) Roofs of factories are painted white or with aluminium paint, because shining roofs are bad radiators of heat, but good reflector of heat.
(B) Metal teapots are kept shining as shining teapots are bad radiators of heat and hence tea remains hot for a long time.
(C) The cooking utensils are kept black from below and shining from the sides, because black surface absorb heat radiation rapidly, but the shining surfaces do not easily radiate heat. Thus, heat is trapped inside the cooking utensil and hence, cooking time is reduced.

 Grow Career Publication
Grow Career Publication
 Param Publication
Param Publication
