- Books Name
- Class 6 Science Book
- Publication
- PathSet Publications
- Course
- CBSE Class 6
- Subject
- Science
Separation of Substances
INTRODUCTION
What is Separation?
The removal of substances from a mixture of two or more substances is known as Separation.
Some Examples of Separation are:
- Separating stones from rice
- Churning milk to obtain butter
On a day-to-day basis, we are faced with various instances when we are required to separate substances from one another. Whether it is picking out chilies from our parathas/poha or separating tea leaves from tea while serving it, the need for separation of substances is something we encounter on a daily basis. It is usually because of one or all three of the following reasons:
To separate two dissimilar but useful elements like in the case of butter and milk. Milk is churned in order to obtain butter.
To segregate useless elements from the useful ones like in the case of separating tea leaves from tea.
To remove and discard impurities or potentially harmful substances like picking out small pieces of stones and other impurities from rice and wheat.

Figure 1 Separating tea leaves from tea
Problems arise when the materials to be separated are really small in size or differ in their composition. It is nearly impossible to separate grains of salt from grains of sand by hand or trying to separate oil from water. We might need to use methods other than simple handpicking even though for a lot of separation processes, even handpicking might be enough.
Why do we need to separate substances?
We carry out the separation of the components of a mixture or an impure substance with the following purposes
- To obtain two different components
- To remove impurities or harmful components
- To obtain the useful component
- To group substances of different sizes
Principle of separation
- The substances present in a mixture retain their original properties like particle size, density, melting point, boiling point, volatility, etc.
- We use the difference in any one of these properties in the components of a mixture to separate them.
What is a Substance?
A substance is a piece of matter with certain features and characteristics.
- Substances characterized into two types as Pure substance and Impure substance.
Pure Substances: The substances which contain only one type of particles.
- Many of the substances we come into contact with only have one type of component particle.
- Pure substances are elements and compounds.
- Iron, copper, water, salt, and other pure substances are Examples.
Impure Substances: The substances which contain more than one kind of particles.
- Impure substances are those that have multiple types of component particles.
- A plate of rice is mixed with stones, Pond water, milk, and other unclean substances are examples.
Impurities:
Impurities are undesired/ unwanted particles in a substance that cause it to be impure.
Element:
Element is a substance made up of the same material's identical particles.
Compound:
Compound is a substance created by the chemical reaction of two or more elements in a specific ratio.
Mixture:
- Mixtures are substances that have more than one component blended in any ratio.
- Air, for example, is made up of a variety of gases such as nitrogen, oxygen, carbon dioxide, dust particles, and so on.
- Mixtures: Substances which contain more than one component mixed in any ratio are called mixtures. For example, air is a mixture of many gases like nitrogen, oxygen, carbon dioxide, dust particles, etc.
- Homogeneous Mixtures: The mixtures in which the particles of the substances present cannot be seen are called homogeneous mixtures. For example, solution of sugar and water, air, cold drinks, etc.
- Heterogeneous Mixtures: The mixtures in which particles of the substances present can be seen easily are called heterogeneous mixtures. For example, water in oil, dust in air.
Solution:
- A mixture of two or more components is referred to as a solution.
- The solvent is the material with the highest concentration, whereas the solute is the substance with the lowest concentration.
- Pure substances are elements and compounds.
Methods of separation
Methods of Separation
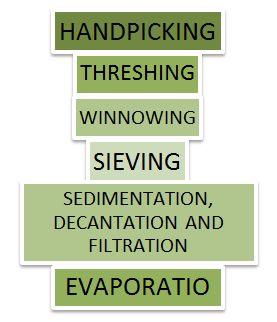
Figure 2 Methods of Separation
- The properties of the components in a mixture, such as particle size, density, melting point, boiling temperature, volatility, and so on, remain unchanged.
- To separate the components of a mixture, use the differences in any one of these qualities.
- The following methods are used for separation, these are as follows:
- Threshing
- Winnowing
- Handpicking
- Sieving
- Magnetic Separation
- Floating and Sinking Method
- Sedimentation and Decantation
- Loading
- Filtration
- Separation to Immiscible Liquids
- Churning to Separate Cream from Milk
- Sublimation
- From above Threshing, Winnowing, Handpicking, Sieving and Magnetic Separation methods are used to separate the solid from other solids.
- Evaporation and Condensation methods are used to separate water soluble solids or soluble solute in the solvent.
- Sedimentation, Decantation, Loading and Filtration methods are used to separate insoluble solids from liquids.
- Funnel, Centrifugation and Churning methods are used for separation of immiscible liquids.
- Floating and Sinking Method and Sublimation methods are used for removing a non-soluble solute from a solvent.
- Handpicking: The simple process of separating slightly bigger sized harmful substances or other useful substances or impurities like small pieces of stones, husk and dirt from grains of wheat, pulses and rice is called handpicking.
- In situations when the quantity of such impurities is not very large, handpicking turns out to be a time-saving and convenient procedure of separating substances.
- Rice, wheat, pulses, etc., that we buy from the market may contain impurities (unwanted or harmful particles) in the form of small stones, unwanted grains, etc. Often, these impurities look very different from the food item and can be spotted easily.
- The method of separation used in such a case is hand-picking (Fig. 3.4). This method is preferred when
- the quantity of the mixture is small,
- the unwanted substance is present in smaller quantities, and
- the size, shape, or color of the unwanted substance is different from that of the useful one.


Figure 3 A group of individuals separating two types of grains
- Threshing:
- Grains or seeds of plants like rice and wheat serve as sources of food. The flour (atta) that is used for making chapattis is made from wheat grains.
- After these crops have been harvested or cut, the grains need to be separated from the stalks (the dried stems). This is done by threshing.
- After the crop is harvested, stalks are left to dry under the sun. A single stalk has some 100 pieces of grain seeds joined to it.
- It is manually impossible to pluck each grain seed which is very small in size from the stalk and hence handpicking as a method of separation does not work here.
- That is why we use a method called threshing to separate these grain seeds.
- The process of beating harvested crops to separate the grains from the stalks is called threshing.
- It is done manually (by hand) or with the help of machines. Manual threshing is done by holding a pile of crop and beating it on a rock or a hard surface (Fig. 3.1).
- This loosens and separates the grain from the stalk. Sometimes, threshing is also done by crushing the harvested stalks using bullocks

Threshing is also done with the help of machines like the combine harvester (Fig. 3.2). Threshed grains may still contain seed coverings and tiny pieces of leaves or stem (collectively called chaff). These are separated by winnowing.

- Winnowing: Even when threshing is done, husk or chaff is still attached to the grain seed and since the size of the two is quite similar, handpicking does not work and neither does threshing. Hence, a method called winnowing can be used.
- Winnowing can be defined as the method of separating lighter husk particles and heavier grain seed components by blowing a current of air through them. The lighter husk particles are carried away by the wind and the grain seeds get separated. This husk can be further used as fodder for the cattle.
- The method used to separate chaff from the grain by wind or blowing air is called winnowing.
The mixture of chaff and grain is taken in a winnowing basket (Fig. 3.3). The farmer stands at a higher level and lets the mixture fall to the ground. - The grain, being heavier, falls almost vertically whereas the lighter chaff is carried away by the wind and forms a separate heap away from the grain.
- The separated chaff is used as fodder for cattle. The direction of the wind plays an important role in the process of winnowing.
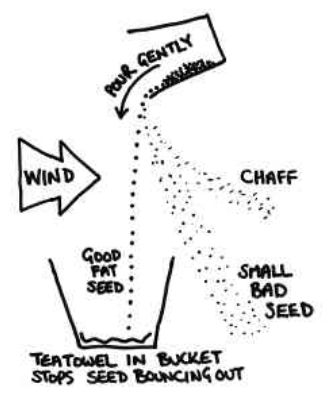
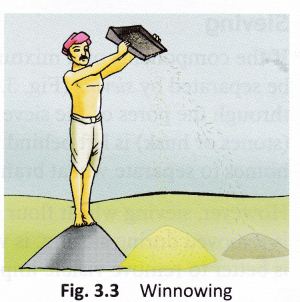
Figure 5 Process of winnowing
- Sieving:
- Sometimes even after the grain seeds have passed through the stages of threshing and winnowing, husk may still be attached to the grain or it may have collected stones and dirt in the earlier stages which need to be removed and this separation is usually done with the help of a sieve.
- Sieving is a very simple, convenient and time-saving process through which particles of varying sizes can be separated from each other with the help of a sieve.
- A sieve is nothing but a simple device with small pores in it which allow finer materials like flour to pass through leaving behind any impurities it might contain.
If the components of a mixture are of different sizes, they can be separated by sieving (Fig. 3.5).- The smaller component passes through the pores of the sieve whereas the larger component (stones or husk) is left behind in it.
- This method is used in some homes to separate wheat bran (the bigger particles) from flour.
- However, sieving wheat flour is not advisable as wheat bran, which is removed during sieving, is very rich in nutrients and is also rich is better to remove visible impurities by hand picking.
- The process of sieving is also used to separate pebbles and stones from sand at construction sites.

The stones and pebbles present in the mixture remain in the sieve and the fine sand particles pass through the holes of the sieve.
Figure 6 Sieving
- Sedimentation, Decantation and Filtration
- Sedimentation: Sedimentation can be defined as the process through which dirt and other heavier particles in a mixture settle at the bottom of the vessel when water is added to it. When the dust and dirt particles have settled, the clear water which forms the upper layer is moved to a different container and the dirt and dust is done away with. This technique can also be used to separate two liquids which do not mix with each other (also called immiscible liquids) and is called decantation.
- Decantation: Decantation can be defined as a technique through which immiscible liquids or a liquid and a solid substance are separated. For example, take the case of oil and water. These are two examples of immiscible liquids. Once we pour oil in water, oil forms the upper layer of water and can be easily separated by gently pouring the mixture in another container till all the oil has been removed. Sometimes smaller dirt particles get carried along with the water in the process of decantation which needs to be further removed. This can be achieved through the process of filtration.
- Filtration: Filtration is the process through which smaller particles like dirt etc. are separated from a solution by making the solution pass through a medium (often a filter paper). This medium is such that only liquids are able to pass through it because of the presence of very tiny pores in it. The filter paper is molded to form a cone and this cone-like structure is then affixed to a funnel through which the dirty solution is allowed to pass. Sometimes, filtration can also be applied to separate pulp and seeds from the juice. It can also be used to separate cottage cheese or paneer from milk.
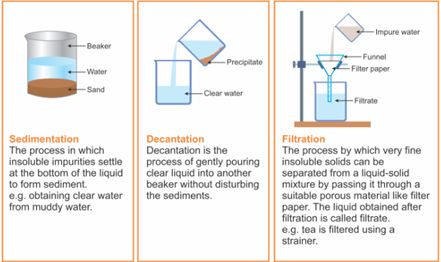
Figure 7 Sedimentation, Decantation and Filtration
- Evaporation: Evaporation is the process of converting liquid into gas or vapour by increasing the temperature or pressure of the liquid. This process is often used to separate salt from salt water or salty sea water. Sea water has a number of salts present in it. Shallow pits called evaporation ponds are constructed and salt water is allowed to stand in these. After some time, the water gets evaporated, leaving behind the salts. Common salt is separated from this mixture upon further purification.

Use of more than one method of separation
Often, we are faced with mixtures and solutions that cannot be separated by use of a single separation technique. A number of such techniques need to be applied simultaneously to achieve the desired result.
Take for example the case of a salt and sand mixture. We know handpicking will not work and considering both of them weigh just about the same, neither will winnowing.
And hence we try to separate the two with the help of filtration or decantation.
We take a beaker and add water to the said mixture of salt and sand. While the salt dissolves in water, the sand deposits at the bottom of the beaker and can be separated from the salt solution with the help of a filter paper or by gently pouring the salt solution in another container. We now have to separate the salt from water, for which we will simultaneously use the methods of evaporation and condensation. While heating the solution in a kettle, we observe that vapour or steam starts to rise from the spout of the kettle. What we then do is allow this steam to come in contact with a metal plate which has some ice on it. When this happens, the steam gets converted to small drops of water which we transfer to another container and thus successfully manage to separate salt which gets left behind in the kettle and the water which we collect in a separate container.
Condensation:
Condensation is the defined as the simple process of converting gas or vapour to its liquid form by decreasing the temperature or pressure exerted on it. This is what we did when we allowed the steam to come in contact with the cold metal plate
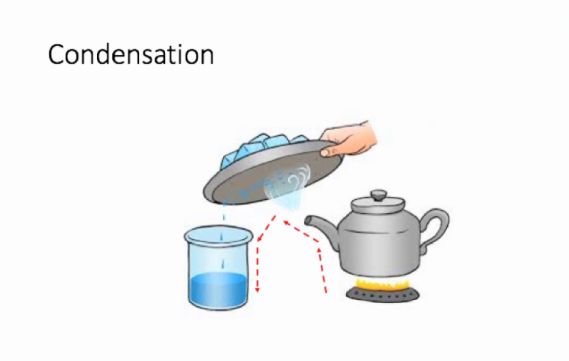
Figure 9 Use of more than one method of separation
Can Water Dissolve Any Amount of a Substance?
Even though water can dissolve a number of substances and solutions in it, it has a limit to how much it can dissolve. After a certain point, it stops dissolving any more of that substance and the substance collects at the bottom of the vessel. We say that the solution has become saturated.
A saturated solution is one that contains the maximum possible concentration of a particular solute. For example, if we continue to add increasing amounts of salt to a small quantity of water, there will come a point that the salt will not get mixed with the water and instead deposit at the bottom. At this point, we say that the solution has become saturated i.e. it is now incapable of dissolving any more of the given solute which is in this case, salt.
A salute is defined as a very small element in a solution that is dissolved in a solution.
One way of ensuring that the given amount of water takes more salt even after it has reached its saturation point is by heating the said water. This is because heating the solution helps to increase the solubility of salt or any solute and hence more amount of the same solute can now be dissolved in the same amount of water.
Some Important Definitions
Churning: The process of shaking milk or cream in order to allow lighter particles to come to the surface in order to make butter is called churning.
Pure Substance: This can be defined as a substance composed of only a single type of particle.
Impure Substance: A substance composed of more than one type of particles.
Sublimation: When a solid directly gets converted into vapour, this process is known as sublimation.
Magnetic Separation: This is another method of separation which allows metals (and other articles which are attracted to a magnet) to be separated from a mixture with the help of a magnetic or by applying a magnetic force to it. For example, a mixture of salt and iron filings can be separated with the help of a magnet.

 Param Publication
Param Publication
 PathSet Publications
PathSet Publications
