- Books Name
- Physics Book Part l and ll
- Publication
- Grow Career Publication
- Course
- CBSE Class 12
- Subject
- Physics
Coulomb’s Law
The force of attraction or repulsion acting along a straight line between two electric charges is directly proportional to the product of the charges and inversely to the square of the distance between them.
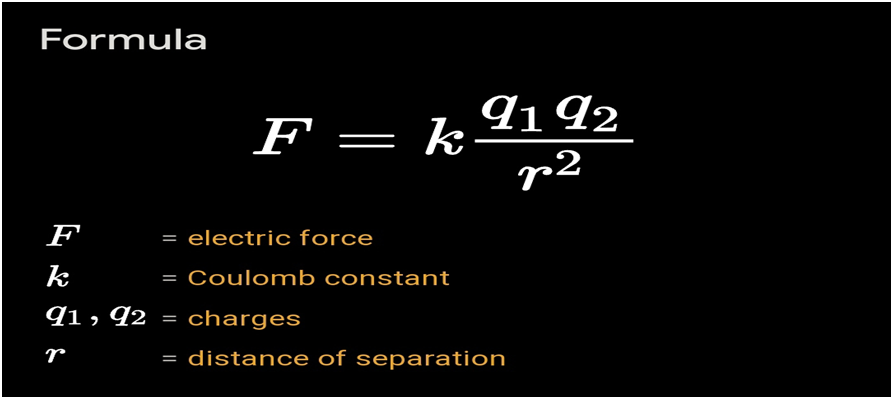
Forces Between Multiple Charges
- When our synthetic clothing or sweater is removed from our bodies, especially in dry weather, a spark or crackling sound appears. With females’ clothing like a polyester saree, this is almost unavoidable.
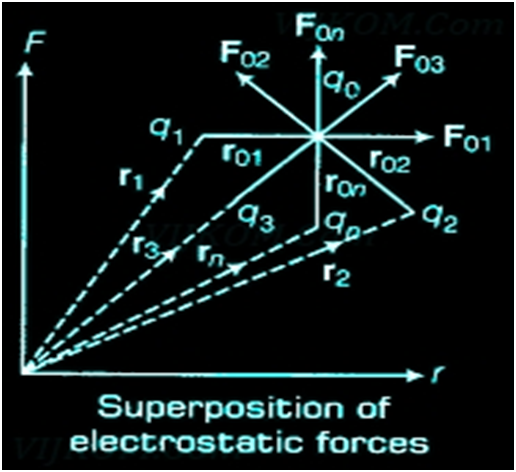
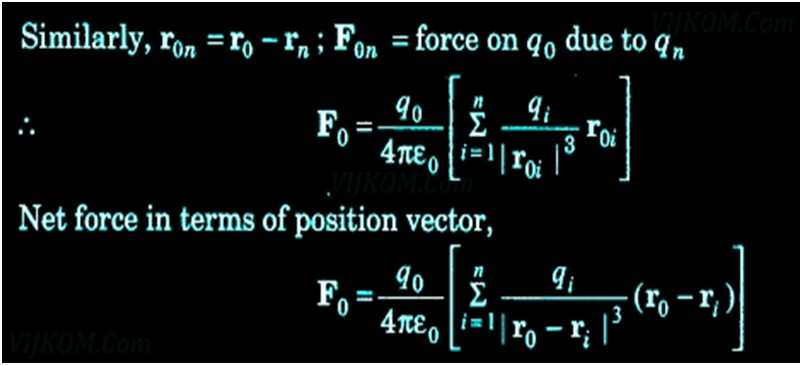
- It does not help to calculate the force on a charge where there are not one but several charges around. It is have been proved via an experiment that force on any charge due to a number of other charges is the vector sum of all the forces on that charge due to the other charges, taken one at a time.
Principle of Superposition of Electrostatic Forces This principle states that the net electric force experienced by a given charge particle q0 due to a system of charged particles is equal to the vector sum of the forces exerted on it due to all the other charged particles of the system.
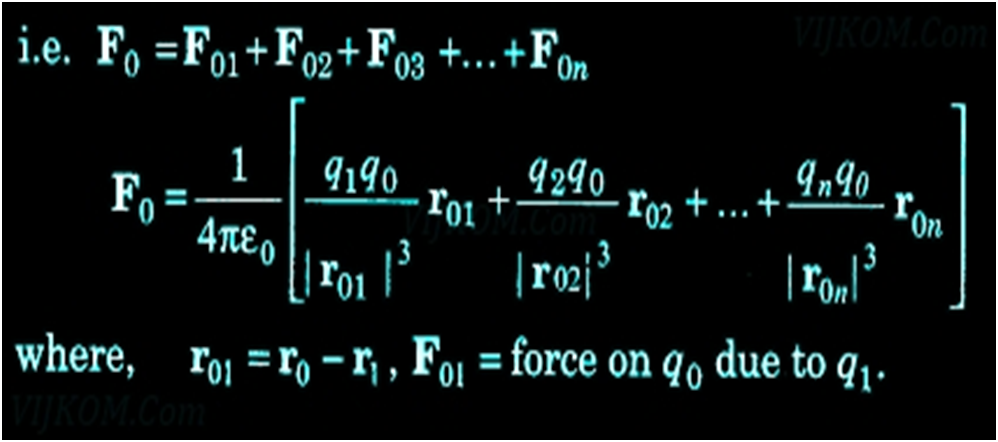
Some basic Properties of Electric Field Lines
- Two-line never cross each other
- These electric field lines start on the positive charge and end in the negative charge
- Electrostatic field lines do not form any closed loops
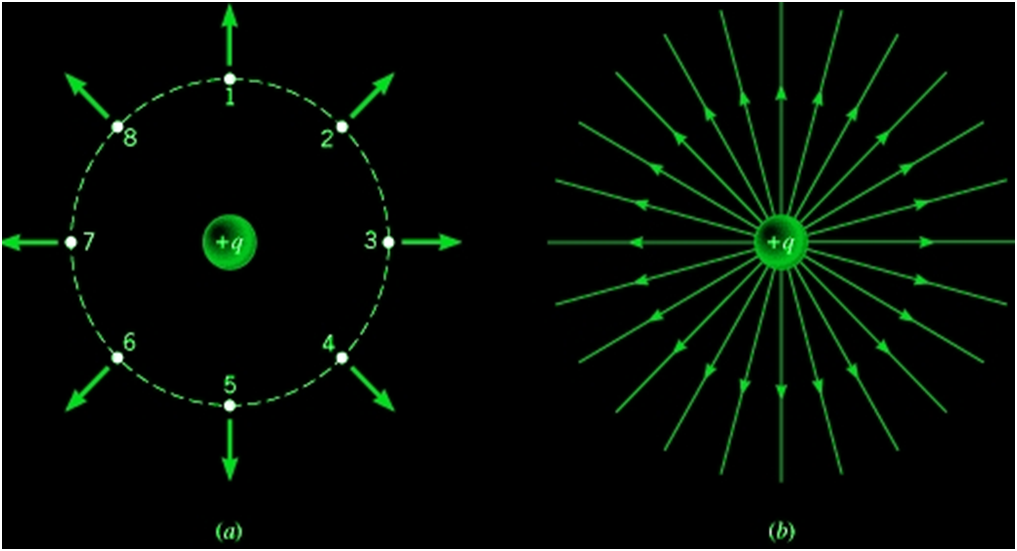
Electric Flux

The total number of electric field lines passing a given area in a unit time is defined as the electric flux.
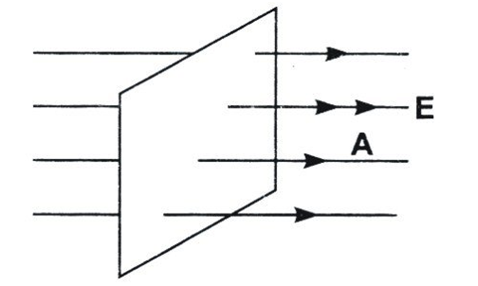
- Since electric field is uniform, it is created by a source very far from the closed surface. Or there is no charge enclosed within the closed surface. Hence, net flux through it is zero.
- This argument does not hold true for an open surface as an open surface can be arbitrarily extended to a closed surface enclosing a non-zero charge in which case the electric flux through the surface may become non-zero
- Electric flux is defined as the measure of count of number of electric field lines crossing an area. Electric flux ϕ=EAcosθ SI unit of electric flux is Nm2/C.
Electric Dipole
Physical significance of dipoles
In most molecules, the centres of positive charges and of negative charges lie at the same place. Therefore, their dipole moment is zero. CO2 and CH4 are of this type of molecules. However, they develop a dipole moment when an electric field is applied. But in some molecules, the centres of negative charges and of positive charges do not coincide. Therefore they have a permanent electric dipole moment, even in the absence of an electric field. Such molecules are called polar molecules. Water molecules, H2O,is an example of this type. Various materials give rise to interesting properties and important applications in the presence or absence of electric field.
Electric dipole
An electric dipole is a pair of equal and opposite point charges -q and q, separated by a distance 2a. The direction from q to -q is said to be the direction of the dipole.
p=q×2a
where p is the electric dipole moment pointing from the negative charge to the positive charge.
EXAMPLE
Force on electric dipole

Dipoles in an External Electric Field
Consider an electric dipole placed in an external electric field. The electric dipole will experience some force and is known as the torque. The torque is the force exerted on the dipoles placed in an external electric field and is
given by,⇒τ = P x E = PE Sin θ ………(1)
Where,
P - The dipole moment
E - The applied external field
Significance of Electric Dipole and Moment
The concept of an electric dipole is not only having importance in physics but it is an equally valid and prominent topic in chemistry as well. We know that most of the matter made up of atoms and molecules will be electrically neutral. Depending upon the behaviour of the pair of charges, the molecules are subdivided into two types
- Polar molecules: If the centre of mass of positive charge doesn’t coincide with the centre of mass of negative charge then it is known as a polar molecule.
- Non-Polar molecules: If the center of mass of positive charge coincides with the center, charges, s of negative charge then it is known as a Non-Polar molecule.

 Madhava Publications
Madhava Publications
 Grow Career Publication
Grow Career Publication
