- Books Name
- Physics by Anshu Physics Book
- Publication
- Madhava Publications
- Course
- CBSE Class 12
- Subject
- Physics
Coulomb’s Law
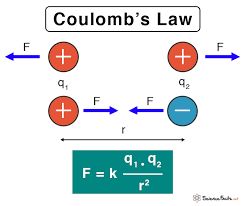
Suppose there is a charge at a point in space if we take another charge near that previous charge. These two charges will experience a force on each other. This force is consistent with Newton's third law which says that there is equal and opposite reaction for every action.
The magnitude of the force is given by Coulomb's law. If you look at Coulomb's law you will notice that it is very similar to the universal law of gravitation. Let's see what Coulomb's law states.
If you have two charges q1 and q2 placed at a distance r from each other then they will experience a force due to one another.
Coulomb's law states that the magnitude of this force is directly proportional to the square of these charges and inversely proportional to the square of the distance between them.
Where |F| is the magnitude of the force between two charges,
r is the distance between the charges
q1, q2 are the magnitude of the charges.
Here K is a proportionality constant whose value is 1/(4*
Here
Coulomb’s force is along the straight line connecting the points of location of the charges and hence coulomb force is central and spherically symmetric.
This force is attractive when the two charges are of different signs and the force is repulsive when two charges are of the same signs
Definition of 1 coulomb:
If q1= q2 = 1C and r = 1 m Then F= K = 9 *10 power 9 N
So one coulomb Is the charge that when placed at a distance of 1 meter from another charge of the same magnitude in vacuum experience and electric force of repulsion of magnitude 9 *10 power9 N.
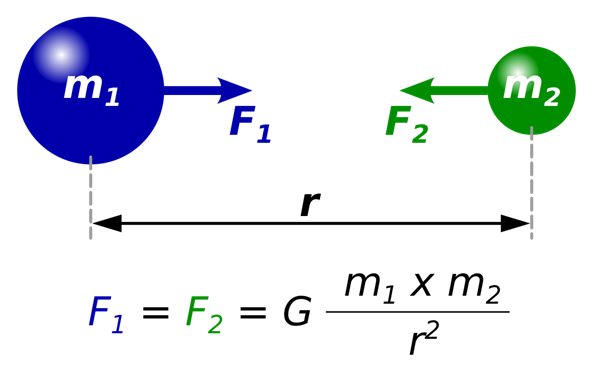
The analogy between Gravitational force and Coulomb’s force
The electrostatic force is the force of attraction or repulsion between two charges at rest while the gravitational force is a force of attraction between two bodies by virtue of their masses.
Similarities:
- Both follow Inverse Square Law i.e, F is proportional to 1/r^2
- Both forces are proportional to the product of masses or charges.
- Both are Central forces in the app along the line joining the centers of two bodies.
- Both are conservative forces; the work done against these forces does not depend upon the path followed.
- Both forces can operate in a vacuum.
- The range of both forces is infinite.
Dissimilarities :
- Gravitational force is attractive while electrostatic force may be attractive or repulsive.
- Gravitational force does not depend on the nature of the medium why electrostatic force depends on the nature of the medium between the two charges.
- Electrostatic forces are much stronger than gravitational force.
How much is the electrostatic force stronger than the gravitational force
From Coulomb’s law if we wish to calculate the force between an electron and a Proton separated by a distance r.
Fe=( k q1*q2)/r^2 , here q1 and q2 are the charges of electron and proton (-e) and (e) respectively.
Fe= k(-e)(e)/ r^2 = -ke^2/r^2
Negative signs show that this force is of attractive kind.
now let's see the gravitational force between an electron and a Proton separated by a distance r.
Fg =G*me*mp/r^2
If you take the ratio of electrostatic force and the gravitational force between electron and proton ( Fe/Fg), you will see that the value of this fraction is of the order of 10power39.
Some examples show how electric forces are enormously stronger than the gravitational forces.
- If you stand on the earth there will be a gravitational force that is pulling you towards its center, but you do not move towards the center.So there must be some other force that is balancing the gravitational force upon you. That force is actually the electrostatic force. So you see that the electrostatic force between the ground and feet is enough to balance the gravitational force due to the entire earth on you.
- When we hold a book against gravity in our hand, the electrostatic force between hand and book is enough to balance the gravitational force on the book due to the whole earth.
The direction of coulomb’s Force
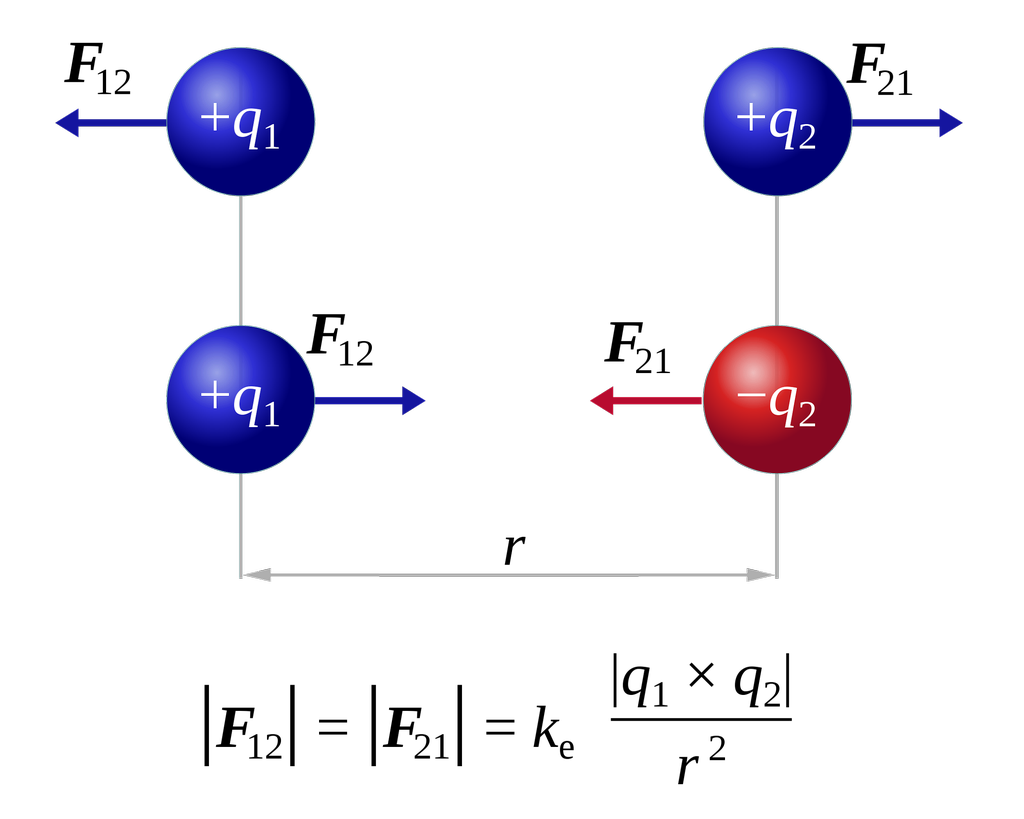
- If we have two charges whose signs are the same either both positive or both negative, then the force will be repulsive and they will try to push Each Other away. As electrostatic force is consistent with Newton's third law the force on the first charge due to the second ( F12 ) is equal to the force on the second charge due to the first (F21). but they are in opposite directions.
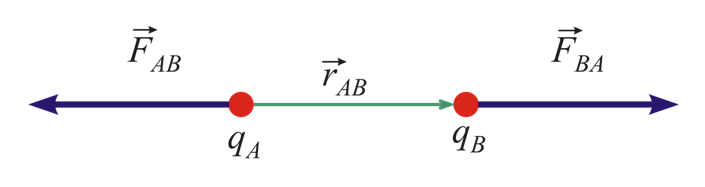
- If you have two opposite charges then they will attract each other, they will try to pull each other.
In the figure given below positive charge is indicated as blue ball and negative charge is indicated as a red ball.You can see that when both charges are positive the forces on one due to other is such that they are pushing the other charge away.
In the second place when one charge is positive and other is negative they try to pull each other as shown.
The magnitude of electrostatic force in both the cases is given by Coulomb’s law and |F21|= |F12| but their directions are opposite.
A fun thing to try
I am providing you the link to a simulation related to electric charges and coulomb’s force.
You can choose the two charges and their sign and it will show you the magnitude of the Force with its direction.
https://phet.colorado.edu/sims/html/coulombs-law/latest/coulombs-law_en.html
Electric force due to Multiple charges: Principle of Superposition.
With Coulomb's law, we can find the force between two charges, But what we will do when we have to calculate force on a charge due to multiple charges, the answer to this lies in a fantastic principle called Principle of superposition.
The principle of superposition states that the force on a charged particle due to multiple charges is actually the vector sum of the forces exerted on it due to all other charges. The force between two charges is not affected by the presence of other charges.
Let me explain this further.
suppose we have five charges ( Q1, Q2, Q3, Q4, Q5) at a distance (d1, d2, d3, d4,d5) from a point L, A charge Q is placed at L.
So Total force on charge Q will be the vector sum of force exerted by all charges .
Force on Q (FQ)= F(QQ1) + F(QQ2) + F(QQ3)+F(QQ4)+ F(QQ5)
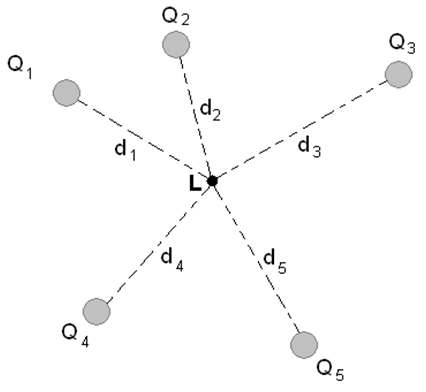
Please note that this is actually a vector sum, we need to add these vectors keeping in mind their directions too.
Eectric Field
We have already discussed the coulomb’s law and the principle of superposition. These two concepts allow us to calculate force one charges due to other charges. The electrostatic force acts between two bodies without even direct contact between them so to visualize how the electrostatic force is experienced by a charge we have introduced the concept of electric fields.
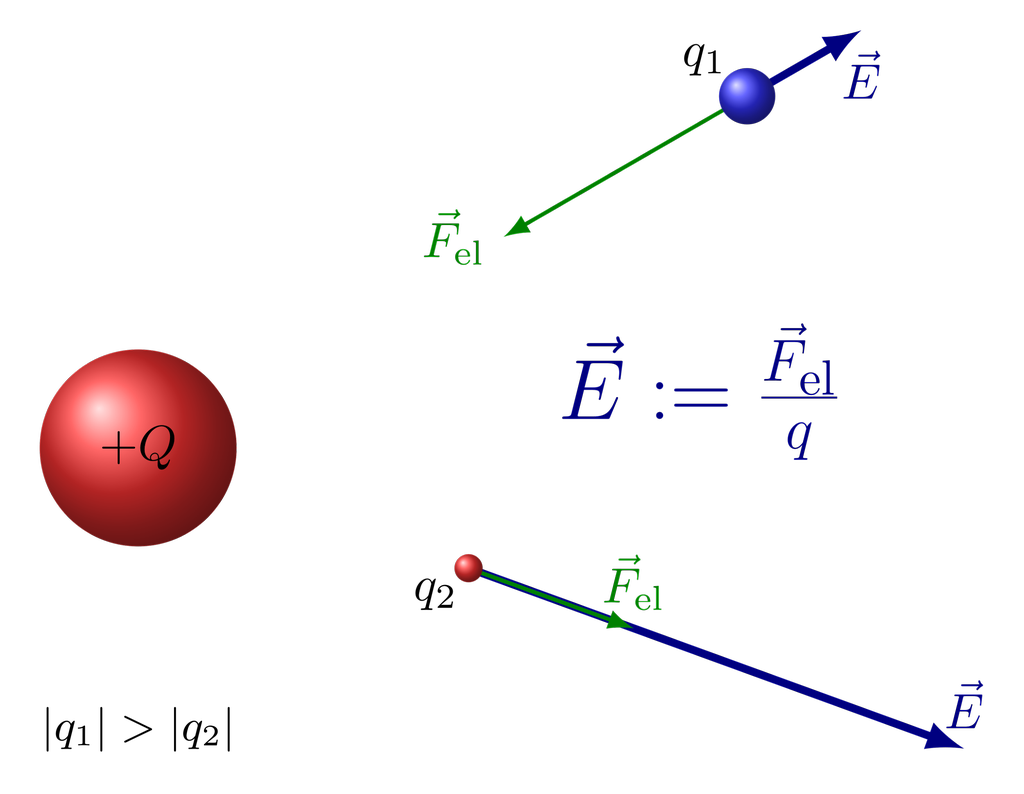
According to this theory when we place a charge Q in space it creates a field around it, specifically the electric field around it which is stretched is space around that charge, and when another charge q is placed in the field of the previous charge it will experience a force on it due to Charge Q.
The magnitude of electric force will be the product of charge “q” and electric field due to charge Q at the position of “q”.
F= q *E due to Q.
Electrostatic force = Charge * Electric field
The Electric field or Electric field intensity for electric field strength E at a point is defined as the force experienced by a unit positive test charge placed at that point without disturbing the position of the source charge.
E= F/q
S.I unit of E= N/C
Direction of Electric field
The electric field is a vector quantity. The direction of the electric field will be same as electric Force for a positive charge but it will be the opposite to electric force for a negative charge
Electric field lines:
Electric field lines are the imaginary lines that are drawn to help in visualizing electric fields. By convention, we take the direction of electric field lines away from the positive charge and toward the negative charge.
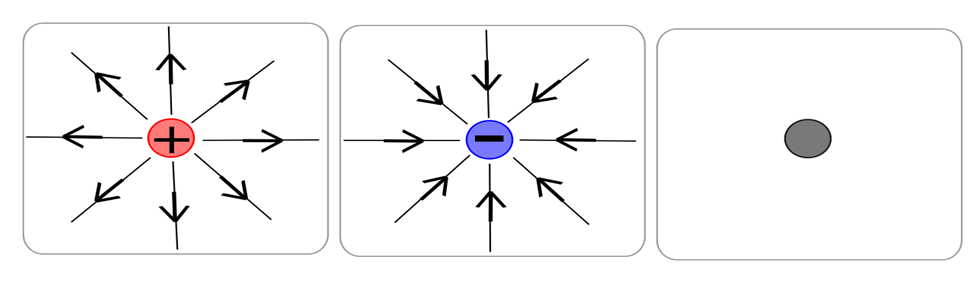
In the above figure electric field lines are shown for positive charge, negative charge and neutral object. There are no electric field lines around the neutral object.
Properties of Electric field lines.
- The line of forces is a continuous smooth curve without any breaks.
- The line of forces starts from positive charge and ends at negative charges.
- They cannot form closed loops
- If there is a single charge then the line of forces will start or end at infinity.
- The tangent to a line of forces at any point will give the direction of the electric field at that point.
- The line of forces can never intersect each other because we would then have two directions of electric field at the point of intersection.
Which is not possible.
- The relative closeness of the lines gives the measure of the strength of the electric field in any region. They are closer together in a strong field and far apart in a weak field.
- Parallel and equispaced lines represent a uniform electric field.
- The line of forces has the tendency to contract lengthwise.
- This explains attraction between two unlike charges.
- The line of forces has a tendency to expand laterally. This explains repulsion between two similar charges.
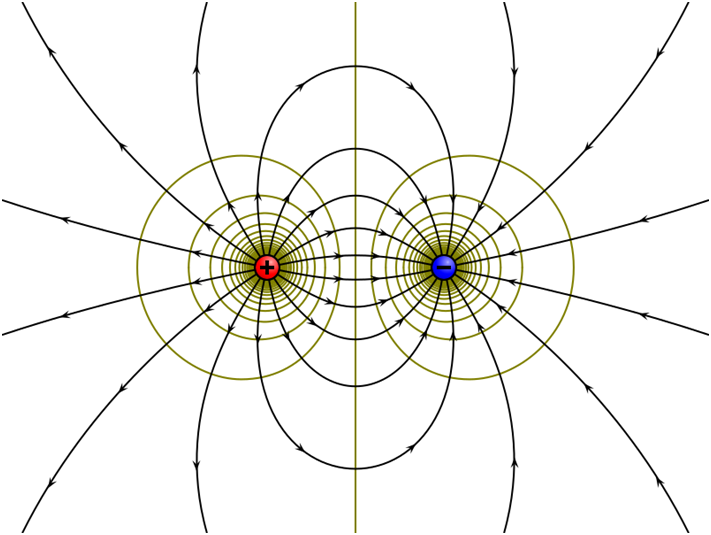
Electric field line due to dipole
Electric dipole is two equal and opposite charges separated by a small distance. The electric field lines start at positive charge and negative charge and they seem to contract lengthwise as if the two charges are being pulled together this explains the attraction between two unlike charges. The figure given below shows the electric field lines due to a dipole.
A fun thing to try and learn.
Below is the link to simulations of electric fields due to charges.
https://phet.colorado.edu/sims/html/charges-and-fields/latest/charges-and-fields_en.htm
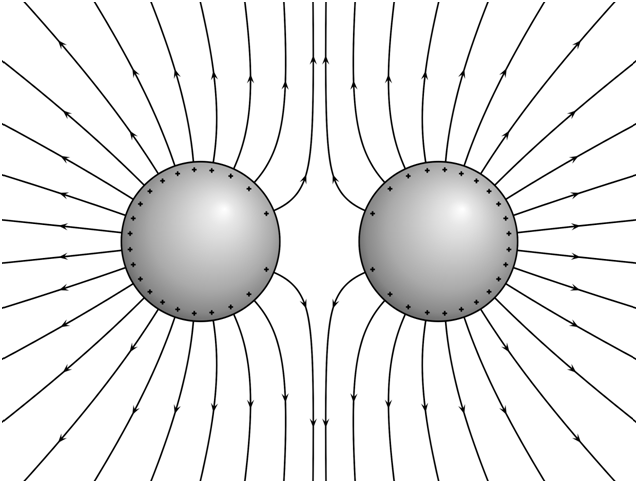
Electric field lines due to Similar charges.
In the figure given below, we have electric field lines due to two positively charged spheres. The field lines start from positive charge and expand laterally as if the two charges are being pushed away from each other. This explains repulsion between two like charges.
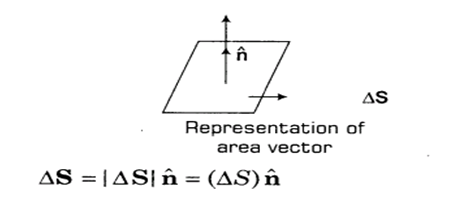
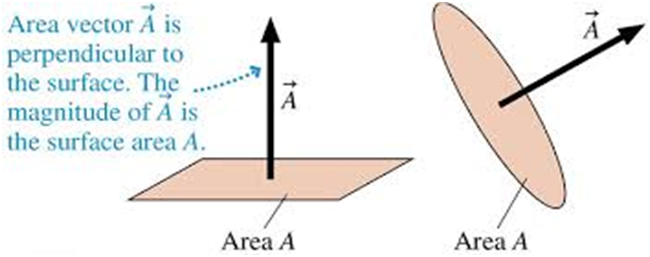
Area Vector.
Suppose we have a disc of radius “r”, So there must be some surface area of this disc. This is actually the magnitude of the area of this plane disc. But we can also define its direction. Area vector is actually perpendicular to the surface.
The direction of a planer area vector is specified as the normal to the plane.
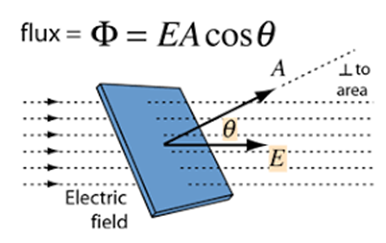
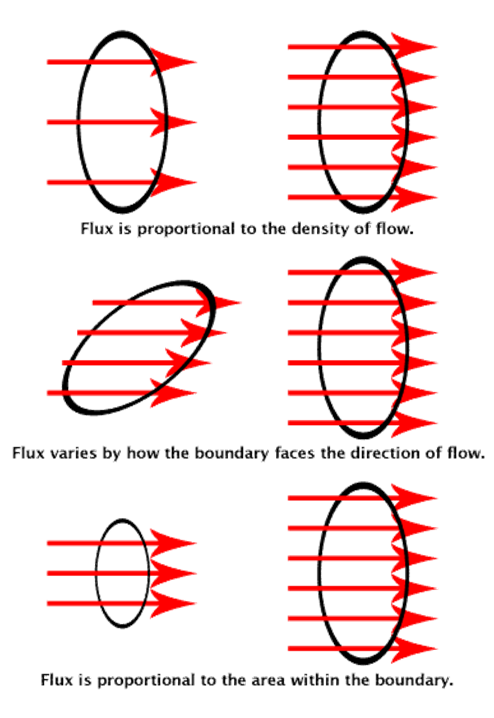
Electric flux.
The term flux implies some kind of flow, This is a property of every vector. Electric flux is a property of the electric field. The electric flux through a closed surface may be thought of as a number of electric lines of Forces that intersect a given area perpendicularly.
The electric field lines directed into a closed surface are considered negative and those directed out of the closed surface are positive.
If there is no net charge enclosed within the closed surface; Electric field lines going into the closed surface are equal to the electric field lines coming out of the surface. Then electric flux through that closed surface will be zero.
As you can see in the diagram above the formula of electric flux; you will see that electric flux depends on Three things.
- The strength of Electric field (E)
- Area of the surface (A)
- The relative orientation of E and area vector, in other words, electric flux is proportional to cosine of angle between E and Area vector.
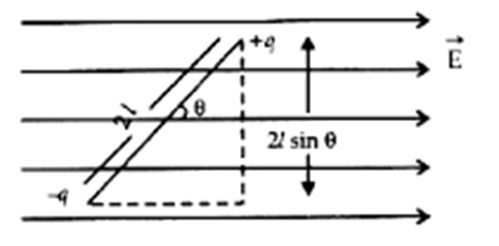
Electric dipole.
Equal and opposite charges (
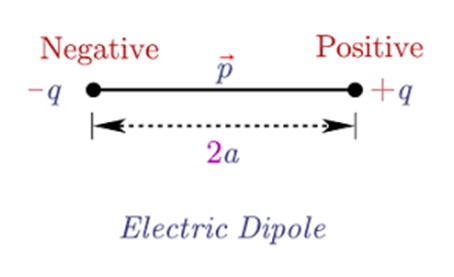
Electric dipole moment “P” = q*d (either charge* distance between them)
The direction of P (electric dipole moment ) is from -q to +q.
All the distances in the electric dipole are measured from the center of the electric dipole.
The electric field at the axial and equatorial position is due to electric dipole.
Axial position:
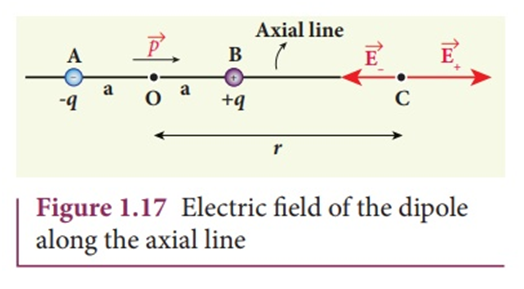
The position in the line of axis of electric dipole is axial position.
Suppose we have a point C along with the axial position of the electric dipole and we wish to find E at point C.
In the figure above if you see the direction of E due to +q it will be away from the charge ( toward right) and direction of E due to -q will be toward the -q charge ( toward left), since the Electric field due to +q and -q points in opposite directions, To find Net field we need to subtract them. E due to +q is greater than E due to -q in the above case as point C is closer to +q and we know that E is inversely proportional to square of distance.
Enet= E(+q) - E(-q) as E(+q)>E(-q) and they are in opposite directions.
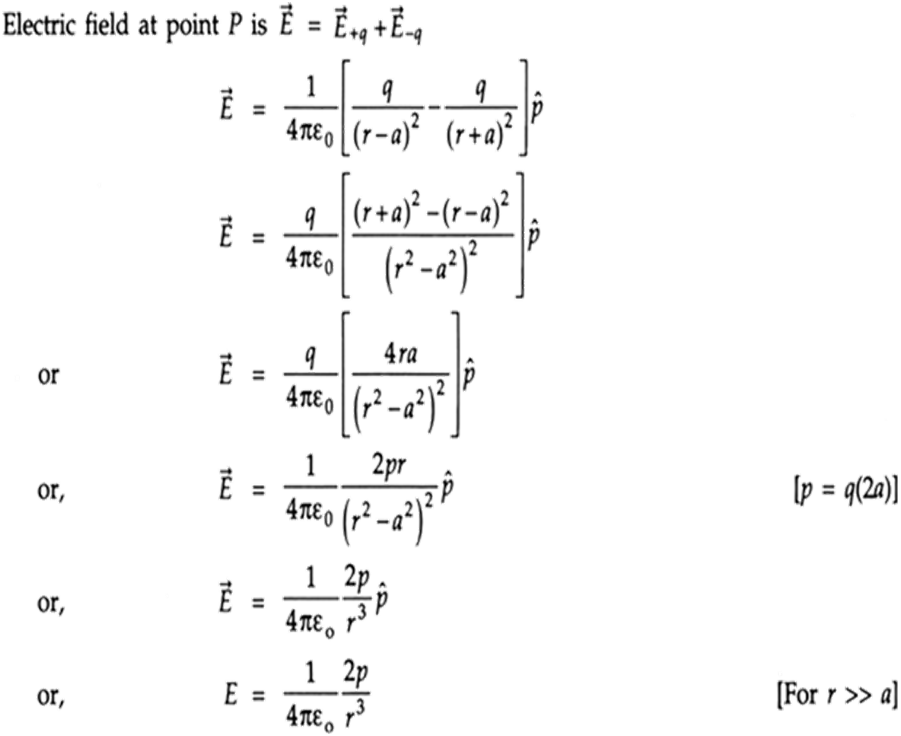
Equatorial position.
The line which is perpendicular to the axis of the dipole and passes through the middle of it is called the Equatorial position.
When we wish to find the Electric field due to the electric dipole at equatorial position P, we first draw the direction of E due to +q and -q at that point P. You can refer to the figure below. Since this E is neither in horizontal direction and nor in vertical, so we must resolve them in horizontal ( cosine components) and vertical directions ( sine components)
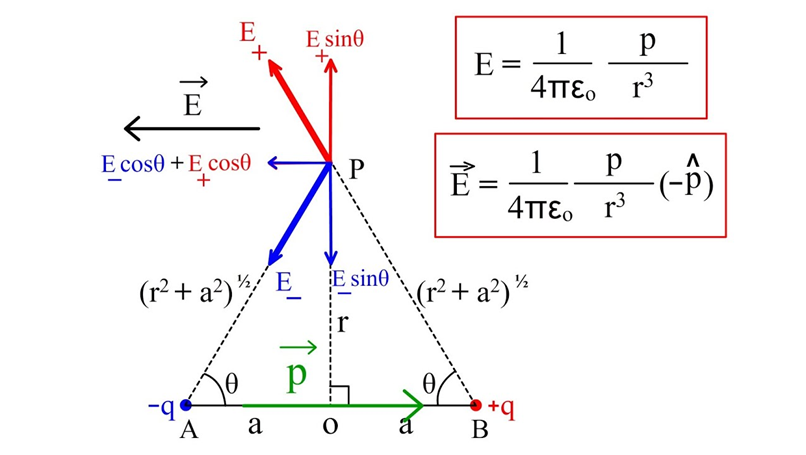
Now you can see that the Sine components of +q and -q are pointing in opposite directions and hence cancel out. And Cosine components of both the charges are pointing toward left ( actually opposite to the direction of electric dipole moment P).
So we conclude that Net E= 2E cos
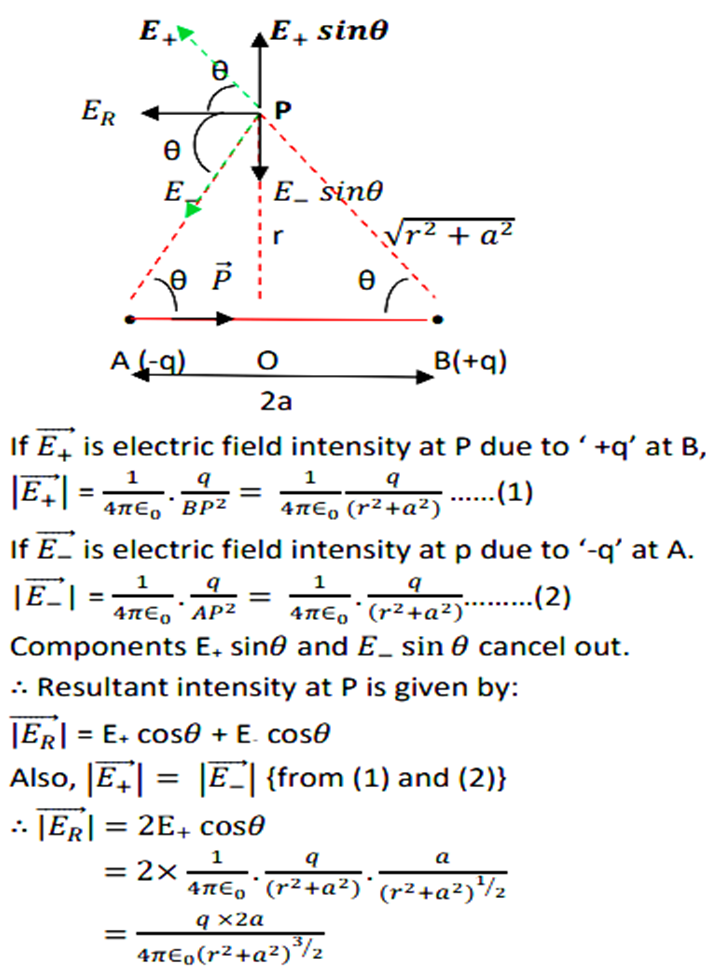
For reference, you can follow the derivation given below.
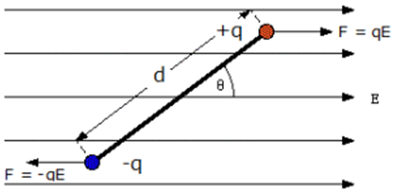
Torque on Electric dipole placed in Uniform ELectric field.
Suppose there is a uniform electric field and an electric dipole is placed at some angle in the uniform field as shown in figure. If we see the direction of force on either charge of the electric dipole we will see that the force on +q and -q will be equal and in opposite direction; Net force on the electric dipole is Zero.
Force = charge * Electric field.
When charge is positive, Force is along the direction of electric field E and when the charge is negative, force is opposite to the direction of E. Since it is the case of uniform Electric field. The value of E at position of (+q) and (-q) will be the same and hence the magnitude of force.
So from above we conclude that Net force on electric dipole is zero.
Torque :
As we have seen, a couple of forces are acting on the electric dipole. Also, there is a separation between these forces, so there must be torque acting on the electric dipole.
Torque = Either force* perpendicular distance between them.
= ( q*E) * (L*sin
Where L= length of the electric dipole
Note that P= qL ( electric dipole moment).
For derivation please refer to the figure below.
Torque= P*E*sin(
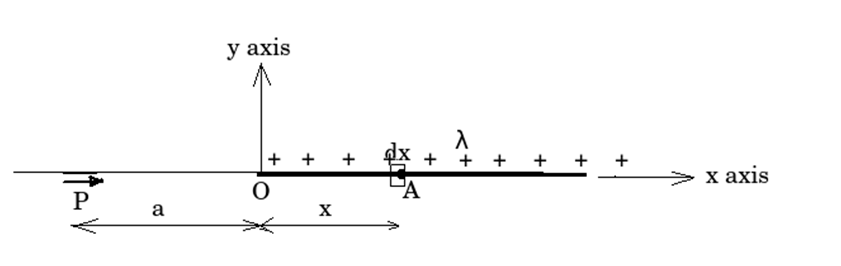
Continuous charge distribution
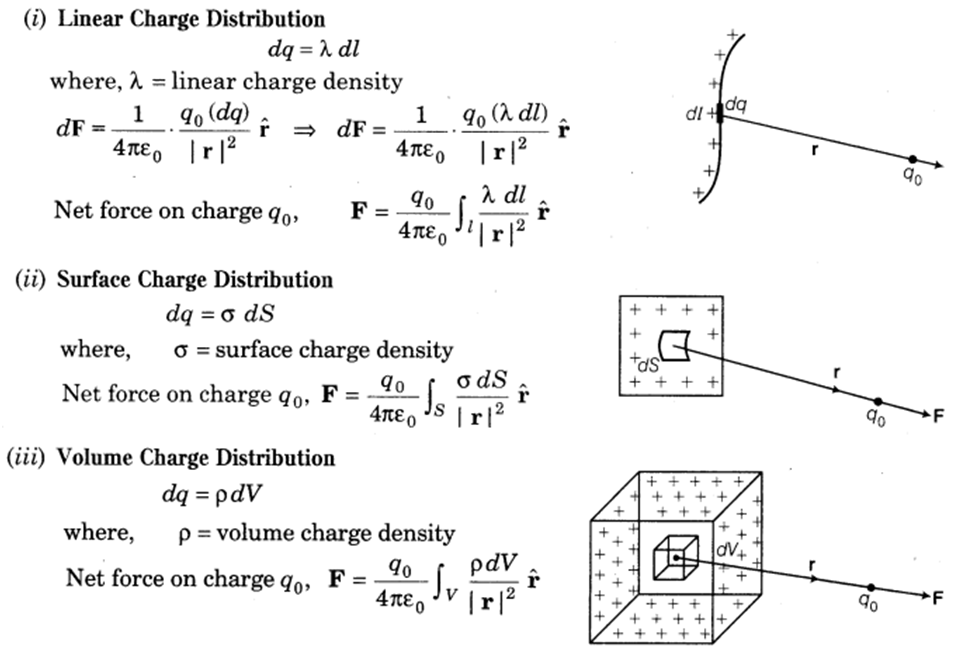
Whatever we have discussed so far, we took the example of point charges or discrete charges. But this is not true every time. The charge can be spread on a line, surface or volume in a continuous manner also.
Different types of continuous charge
- Line charge: when charges are spread in a continuous manner on a line ( 1 - Dimension) we call it line charge distribution.
We define line charge density
- Surface charge: When charges are spread in a continuous manner on a surface ( 2-dimension). We call it surface charge distribution. We define surface charge density at any point of the surface as the charge per unit area at that point. S.I. unit of surface charge density is C/m^2.
- Volume charge density: when charges are spread in a continuous manner in a volume ( 3-dimension), we call it volume charge distribution.
- The volume charge density at any point is given by the charges per unit volume at that point. S.I. unit of volume charge density is C/m^3.
- Books Name
- Physics Book Part l and ll
- Publication
- Grow Career Publication
- Course
- CBSE Class 12
- Subject
- Physics
Coulomb’s Law
The force of attraction or repulsion acting along a straight line between two electric charges is directly proportional to the product of the charges and inversely to the square of the distance between them.
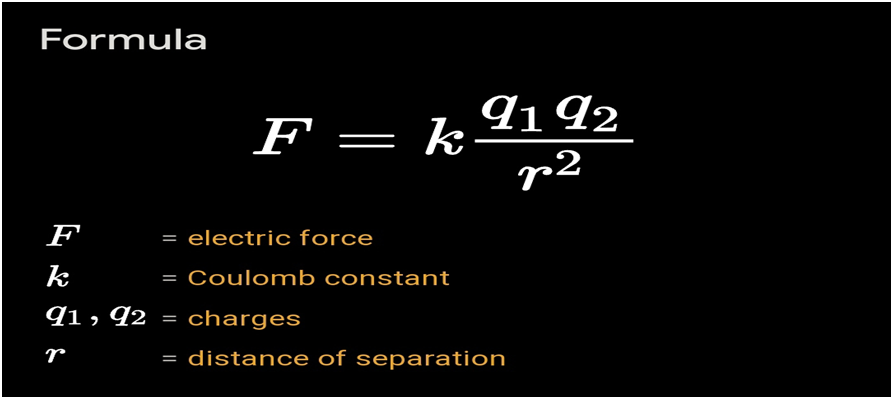
Forces Between Multiple Charges
- When our synthetic clothing or sweater is removed from our bodies, especially in dry weather, a spark or crackling sound appears. With females’ clothing like a polyester saree, this is almost unavoidable.
- It does not help to calculate the force on a charge where there are not one but several charges around. It is have been proved via an experiment that force on any charge due to a number of other charges is the vector sum of all the forces on that charge due to the other charges, taken one at a time.
Principle of Superposition of Electrostatic Forces This principle states that the net electric force experienced by a given charge particle q0 due to a system of charged particles is equal to the vector sum of the forces exerted on it due to all the other charged particles of the system.
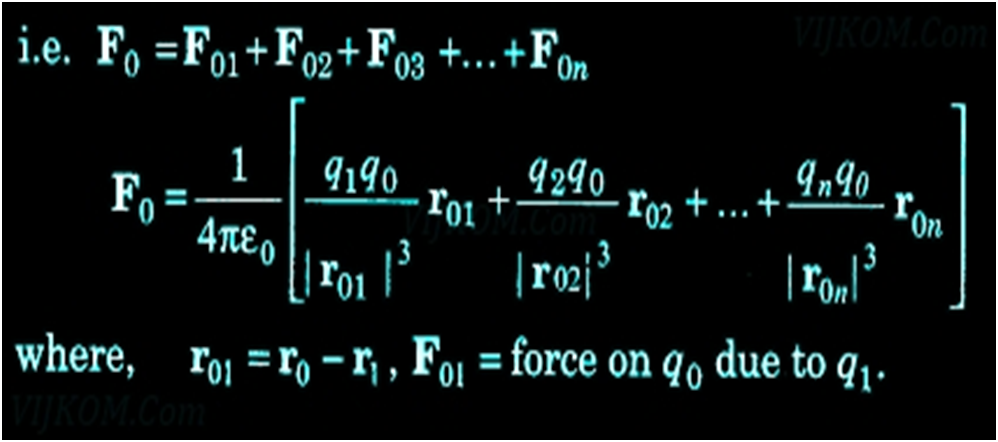
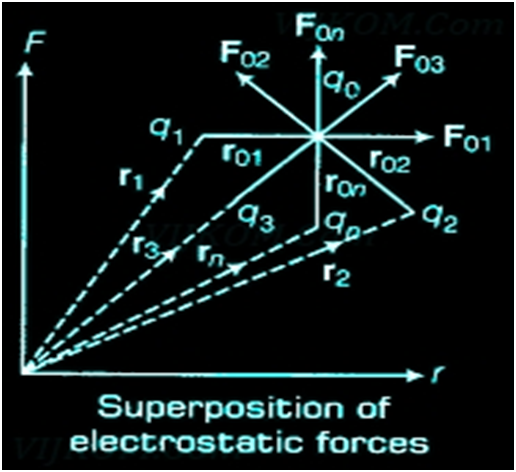
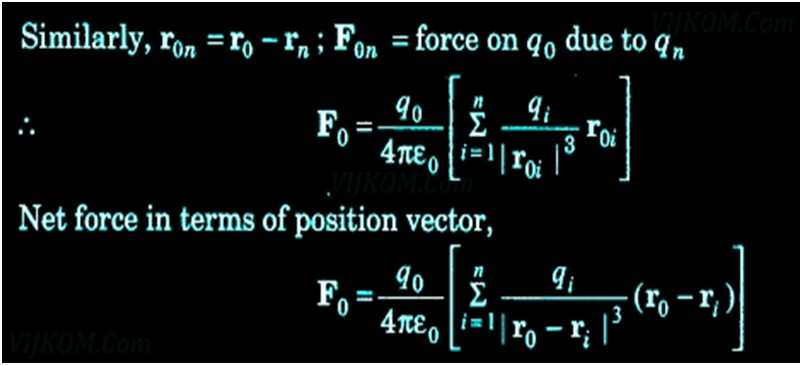
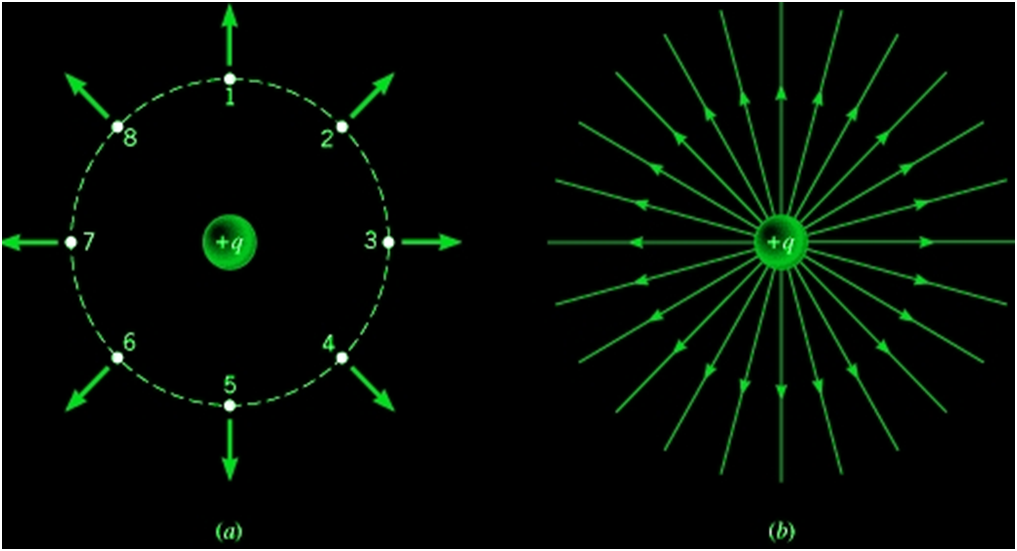
Some basic Properties of Electric Field Lines
- Two-line never cross each other
- These electric field lines start on the positive charge and end in the negative charge
- Electrostatic field lines do not form any closed loops
Electric Flux

The total number of electric field lines passing a given area in a unit time is defined as the electric flux.
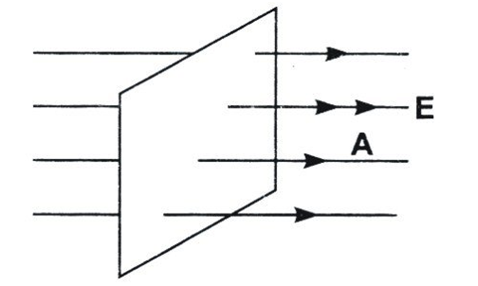
- Since electric field is uniform, it is created by a source very far from the closed surface. Or there is no charge enclosed within the closed surface. Hence, net flux through it is zero.
- This argument does not hold true for an open surface as an open surface can be arbitrarily extended to a closed surface enclosing a non-zero charge in which case the electric flux through the surface may become non-zero
- Electric flux is defined as the measure of count of number of electric field lines crossing an area. Electric flux ϕ=EAcosθ SI unit of electric flux is Nm2/C.
Electric Dipole
Physical significance of dipoles
In most molecules, the centres of positive charges and of negative charges lie at the same place. Therefore, their dipole moment is zero. CO2 and CH4 are of this type of molecules. However, they develop a dipole moment when an electric field is applied. But in some molecules, the centres of negative charges and of positive charges do not coincide. Therefore they have a permanent electric dipole moment, even in the absence of an electric field. Such molecules are called polar molecules. Water molecules, H2O,is an example of this type. Various materials give rise to interesting properties and important applications in the presence or absence of electric field.
Electric dipole
An electric dipole is a pair of equal and opposite point charges -q and q, separated by a distance 2a. The direction from q to -q is said to be the direction of the dipole.
p=q×2a
where p is the electric dipole moment pointing from the negative charge to the positive charge.
EXAMPLE
Force on electric dipole

Dipoles in an External Electric Field
Consider an electric dipole placed in an external electric field. The electric dipole will experience some force and is known as the torque. The torque is the force exerted on the dipoles placed in an external electric field and is
given by,⇒τ = P x E = PE Sin θ ………(1)
Where,
P - The dipole moment
E - The applied external field
Significance of Electric Dipole and Moment
The concept of an electric dipole is not only having importance in physics but it is an equally valid and prominent topic in chemistry as well. We know that most of the matter made up of atoms and molecules will be electrically neutral. Depending upon the behaviour of the pair of charges, the molecules are subdivided into two types
- Polar molecules: If the centre of mass of positive charge doesn’t coincide with the centre of mass of negative charge then it is known as a polar molecule.
- Non-Polar molecules: If the center of mass of positive charge coincides with the center, charges, s of negative charge then it is known as a Non-Polar molecule.

 Madhava Publications
Madhava Publications
 Grow Career Publication
Grow Career Publication
