- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
CRYSTALS DEFECTS (POINT DEFECTS)
Imperfection can be because of :
– Conditions under which crystals have been developed.
– Impurities
– Temp (because of thermal conductivity some atoms/ions can get displaced
These imperfections can be
(a) Point defects – defects will be only at certain lattice positions.
(b) Line defects – If atoms/ions are misplaced/missing/replaced by some other ions along a line
(c) Plane (screw) defects – If atoms/ions are misplaced/missing/replaced by some other ions along a line in a plane.
Types of point defects
Point defect can be classified into three types :
(a) stoichiometric defects
(b) impurity defects
c) non-stoichiometric defect
(a) Stoichiometric defect
These are the point defects that do not distrub the stoichiometry of the solid. They are also called intrinsic ot thermodynamic defects. basically these are two types. Vacancy defecs and interstitial defects.
(i) Vacancy defect : When some of the lattice site are vacant, the crystal is said to have vacancy defect. This results in decrease in density of the substance. This defect can also develop when a substance is heated.
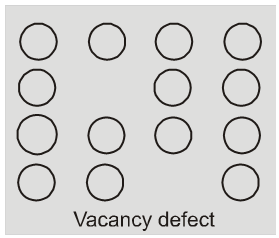
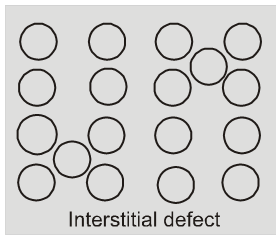
(ii) Interstitial defect : When some constituent particles (atoms or molecules)
occupy an interstitial site. the crystal is said to have interstitial defect. This defect increases the density of the substance.
Vacancy and interstitial defects as explained above can be shown by non ionic solids. Ionic solids must always maintain electrical neutrality.
Rather than simple vacancy or interstitial defects, they show these defects as Frenkel and schottky defects.
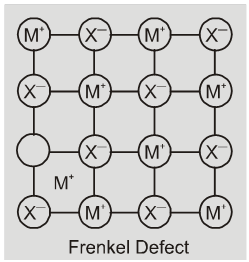
(iii) Frenkel defect : This defect is shown by ionic solids. The smaller ion
(usually cation) is dislocated from its normal site to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location.
Frenkel defect is also called dislocation defect. It does not change the density of the solid. Frenkel defect is shown by ionic substance in which there is a large difference in the size of ions, for example, ZnS, AgCl,AgBr and AgI due to small size of Zn2+ and Ag+ ions.
Eg. ZnS, AgCl, AgBr, AgI etc.
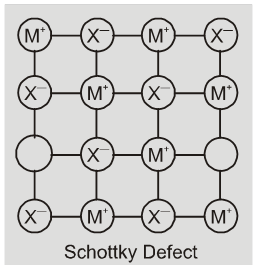
(iv) Schottky defect : It is basically a vacancy defect in ionic solids. In order
to maintain electrical netrality. The number of missing cations and anions are equal.
Like simple vacancy defect, schottky defect also decreases the density of the substance, Number of such defects in ionic solids is quite significant. For example, in NaCl there are approximately 106 schottky pairs per cm3 at room temperature. In 1 cm3 there are about 1022 ions. Thus, there is one schottky defect per 1016 ions. Schottky defect is shown by ionic substance in which the cation and anion are of almost similar sizes. For example,NaCl, KCl, CsCl and AgBr.
Note : It may be noted that AgBr shows both, Frenkel as well as schottky defects.
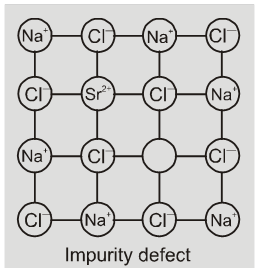
(b) Impurity defects
If molten NaCl containing a little amount of SrCl2 is crystallised, some of
the sites of Na+ ions are occupied by Sr2+. Each Sr2+ replaces two Na+ ions. It occupies the site of one ion and the other site remains vacant. The cationic vacancies thus produced are equal in number is the solid solution of CdCl2 and AgCl.
(c) Non-stoichiometric defect
The defects discussed so far do not disturb the stoichiometry of the crystalline substance. However a large number of non-stoichiometric inorganic solids are known which contain the constituent elements in non-stoichiometric ratio due to defects in their crystal structures. These defects are of two types :
(i) metal excess defect and (ii) metal deficiency defect.
(i) metal excess defect
(a) metal excess defect due to anionic vacancies : Alkali halides like NaCl and KCl show this type of defect. When crystals of NaCl are heated in an atmosphere of sodium vapours, the sodium atoms are deposited on the surface of the crystal. The Cl– ions diffuse to the surface of the crystal and combine with Na atoms to give NaCl. This happens by loss of electron by sodium atoms to form Na+ ions. The released electrons diffuse into the crystal and occupy anionic site. As a result the crystal and now has an excess of sodium. The anionc sites occupied by unpaired electrons are called F-centres (from german word farbenzenter for colour centre). They impart yellow colour to the
crystals of NaCl. The colour results by excitation of these electrons when they absorb energy from the visible light falling on the crystals.
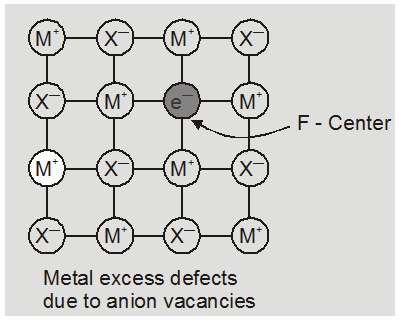
Eg. :
* The excess sodium in NaCl makes the crystal appears yellow.
* Excess potassium in KCl makes it violet.
* Excess lithium in LiCl makes it pink.
Greater the number of F-centres greater is the intensity of colour. This type of defects are found in crystal which are likely to possess schottky Defects.
(b) metal deficiency defect due to the presence of extra cations at interstitial sites : Zinc oxideis white in colour at room temperature. On heating it loses oxygen and turns yellow.
![]()
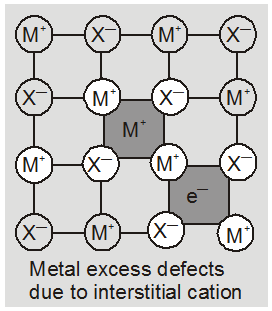
Now there is excess of zinc in the crystal and its formula becomes Zn1+xO. The excess Zn2+ ions move to interstitial sites and the electrons to neghbouring interstitial sites.
(ii) metal deficiency defect : There are many solids which are difficult to prepare in the stoichiometric composition and contain less amount of the metal as compared to the stoichiometric proportion. A typical example of this type is FeO which is mostly found with a composition of Fe0.95O. it may actually range from Fe0.93O to Fe0.96O. In crystals of FeO some Fe2+ cations are missing and the loss of positive charge is made up by the presence of required number of Fe3+ ions.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
