- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chemical Reactions
Nucleophilic addition reactions :
Addition of a nuceophile and a proton across the (C = O) double bond. The reactivity of the carbonyl group arises from the electronegativity of the oxygen atom and the resulting polarization of the carbon-oxygen double bond. The electrophilic carbonyl carbon atom is sp2 hybridized and flat, leaving it relatively unhindered and open to attack from either face of the double bond.
Mechanism :
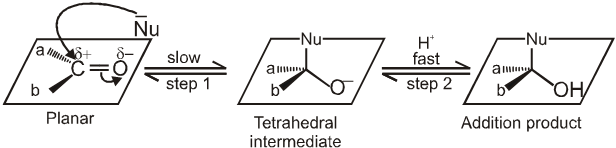
Nucleophile (Nu-) attacks the carbonyl group perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon.
In the process, hybridisation of carbon changes from sp2 to sp3.
A tetrahedral alkoxide is formed as intermediate.
Reactivity : Aldehydes are more reactive than ketones in nucleophilic addition reactions.
![]()
There are two factors which influence the reactivity of ketone and aldehyde.
(i) Inductive effect (ii) steric factor
(i) + I effect of alkyl group decrease the amount of charge on C+ (C+ – O–). in ketones.
ii) Steric effect also causes the less reactivity of carbonyl group.
(I) Addition of hydrogen cyanide (HCN)
![]()
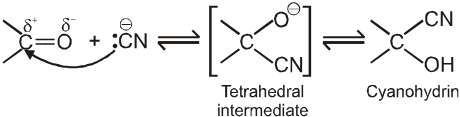
Note :
(i) Addition of HCN over aldehyde and ketones gives cyanohydrin.
(ii) Cyanohydrin on acid hydrolysis gives a-hydroxy acid.
(iii) Cyanohydrin on treating with NH3(l) followed by acid hydrolysis gives a-amino acid.
(iv) In case of ketone cyanohydrin formation is reversible due to bulky group of ketone which hinder the formation.
(II) Addition of sodium hydrogen sulphite (NaHSO3)
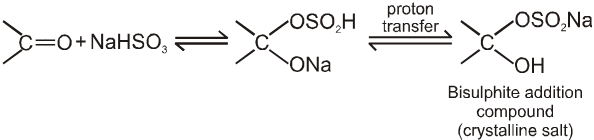
salt on hydrolysis gives carbonyl compounds again, this reaction is used to separate the aldehydes from mixture.
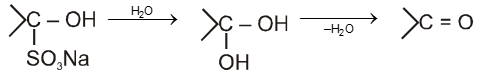
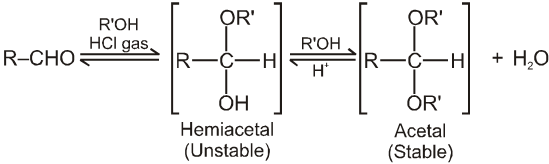
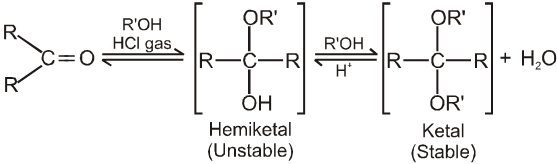
(III) Addition of alcohols (ROH) :
Note :
(i) Acetal is formed to protect aldehyde for a long time.
(ii) Acetal has functional groups ether.
(iii) Acetal formed can be decomposed to original aldehyde by dilute acid.
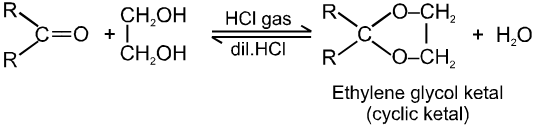
(iv) On treating with ethyleneglycol we get cyclic acetal or ketal.
(v) Acetal formation is found to be more favourable than ketal formation if both the carbonyl groups are present within the molecule.
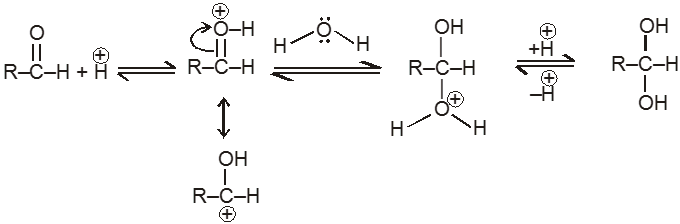
(IV) Addition of water :
Aldehyde or ketone reacts with water to form gem-diols. Water is a poor nucelophile and therefore adds relatively slowly to the carbonyl group, but the rate of reaction can be increased by an acid catalyst.
Mechanism:
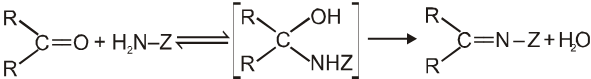
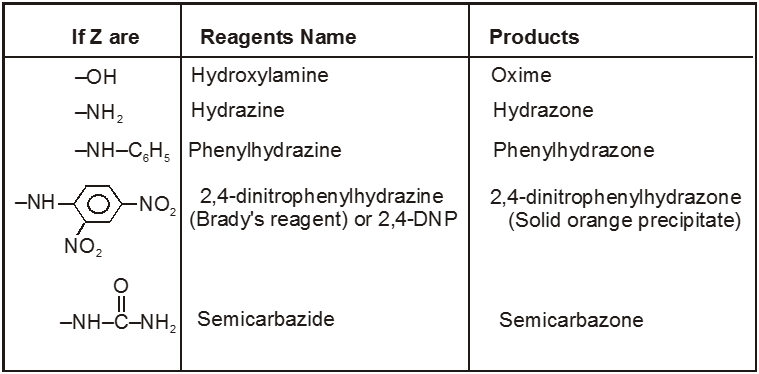
(V) Addition of ammonia and its derivatives (addition elimination reactions) :
(VI) Addition of Grignard reagents (Preparation of alcohol) :
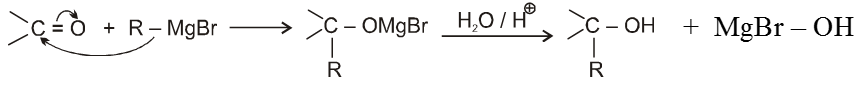
(a) When formaldehyde is treated with Grignard reagent followed by acid hydrolysis primary alcohol is obtained.
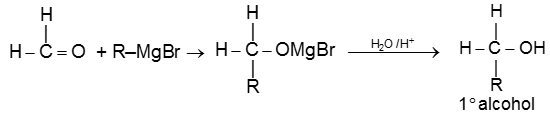
(b) When aldehyde except formaldehyde is treated with grignard reagent followed by hydrolysis 2° alcohol is obtained.
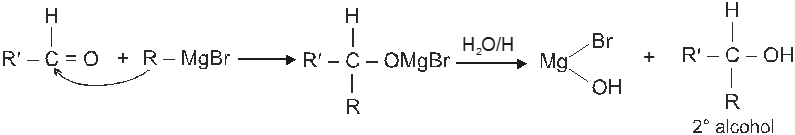
(c) When ketone is treated with grignard reagent followed by acid hydrolysis 3° alcohol is obtained.
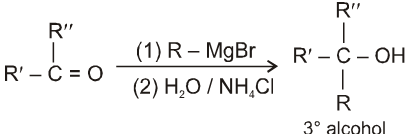
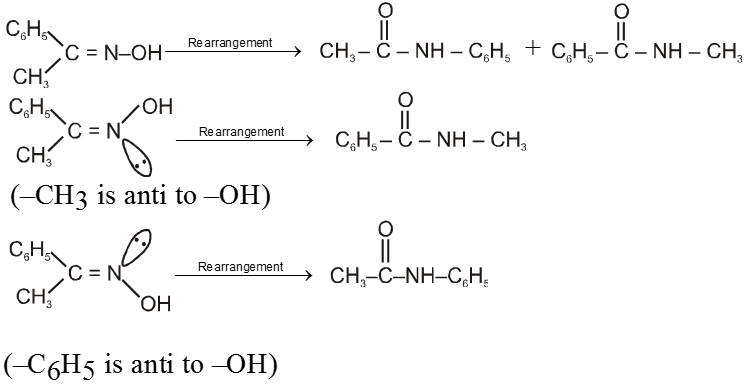
Beckmann rearrangement in Oximes:
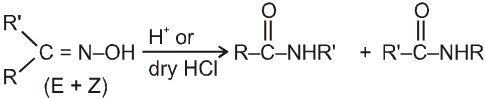 (If R' is bulkier than R)
(If R' is bulkier than R)
Mechanism :
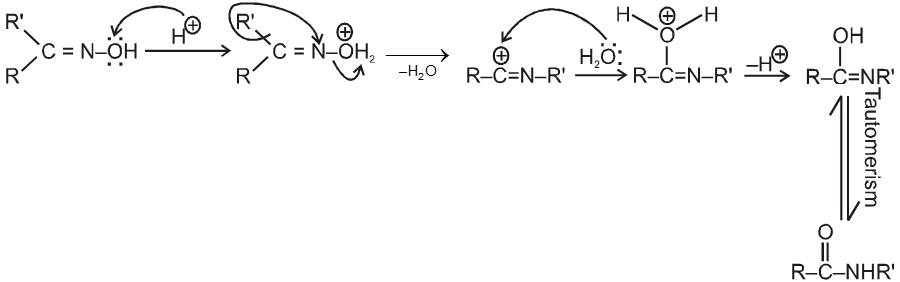
Note :
(i) Oxime undergoes Beckmann rearrangement to give its isomer amide.
(ii) In this reaction the group which is anti to –OH group migrates.
Ex.
Reactions due to a-Hydrogen
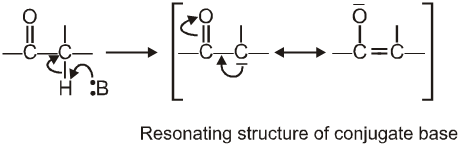
a-Hydrogen of aldehydes and ketones are acidic: They undergo a number of reactions due to the acidic nature of a-hydrogen.
Reason for the acidity of a-hydrogen: Strong electron-withdrawing effect of the carbonyl group, and resonance stabilisation of the conjugate base
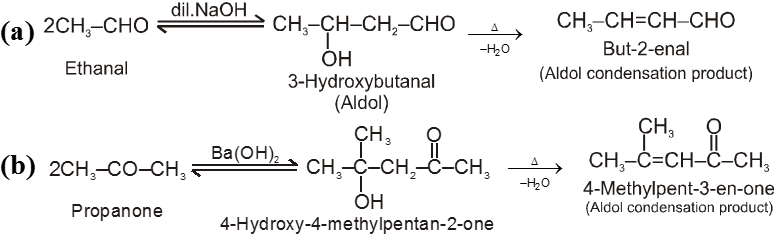
(I) Aldol condensation (or aldol reaction)
Aldehydes and ketones with at least one a-hydrogen undergo a reaction in the presence of dilute alkali as catalyst.
Mechanism
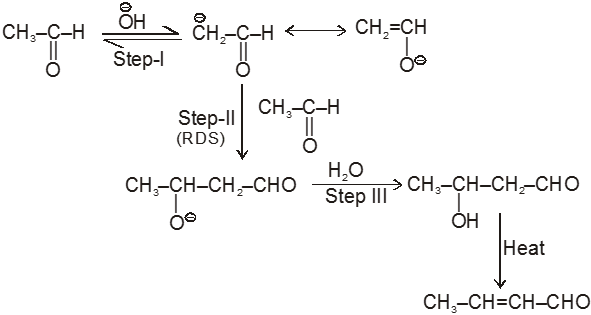
Ex.
(II) Cross-Aldol condensation :
On using two types of carbonyl compounds both having a-hydrogen atoms we get a mixture of four condensed product because two types of carbonyl compounds will give two type of carbanions which will be nucleophile for itself and other molecule.
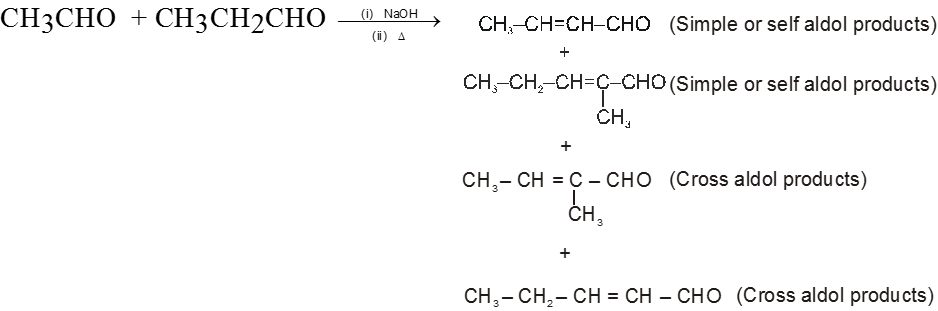
Ketones can also be used as one component in cross-aldol reactions.
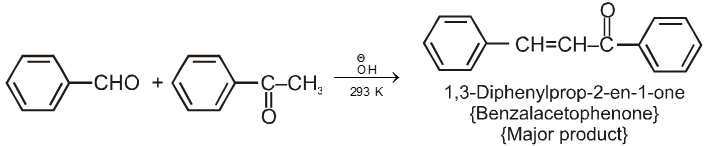
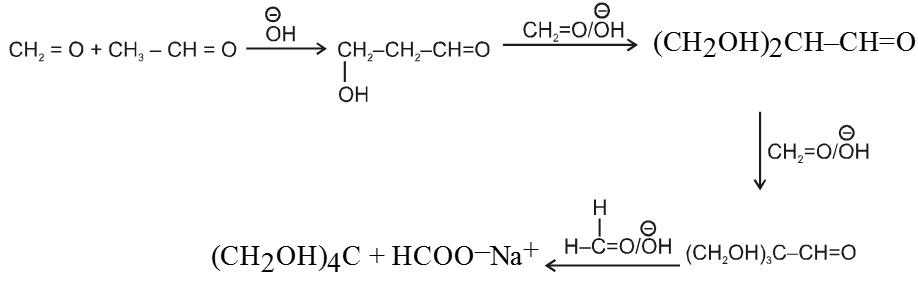
Note : On using formaldehyde and acetaldehyde during crossed aldol all the a-hydrogen atom of acetaldehyde are replaced one by one by hydroxymethyl group because of smaller size of formaldehyde to give trihydroxymethylacetaldehyde which undergoes crossed cannizaro's reaction with formaldehyde to give tetrahydroxymethyl methane and formate ion as a final product.
Ex. Show how cinnamaldehyde is prepared by crossed aldol condensation ?
Sol. ![]()
(III) Intramolecular aldol condensation :
If two carbonyl groups with a-hydrogen atoms are present within the same molecule, then we get cyclic a, b-unsaturated aldehyde / ketones via the formation of cyclic-b-hydroxy aldehyde / ketone in presence of basic medium.
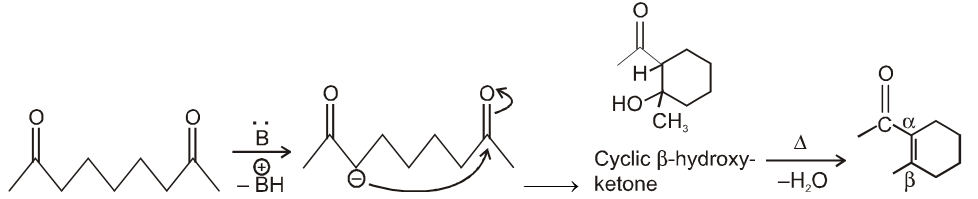
By knowing product we can get reactant as in case of intermolecular aldol condensation :
Perkin reaction :
When aromatic aldehyde like benzaldehyde is treated with anhydride in the presence of sodium salt of acid from which anhydride is derived we get a, b-unsaturated acid.

Mechanism :
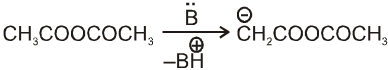
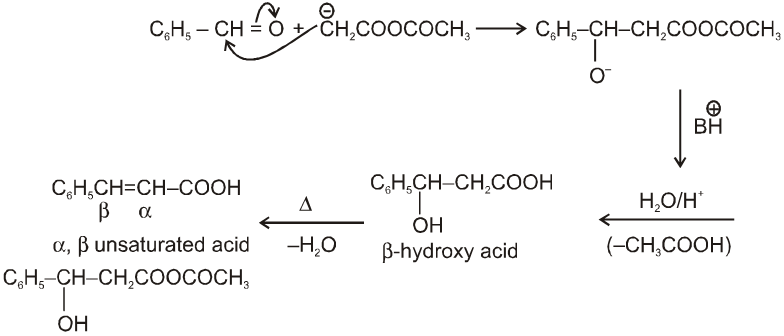
Note : By knowing a, b-unsaturated acid we can get idea about the anhydride used in perkin reaction. This can be done by keeping 'H' at a and –OH at b-carbon atom followed by breaking a, b- carbon. By this we can know about acid and it will be anhydride of this acid only.
Cannizzaro reaction :
Aldehydes which do not have an a- hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on treatment with a concentrated alkali.
Ex.
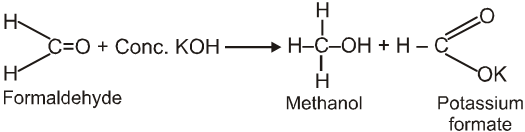
Mechanism :
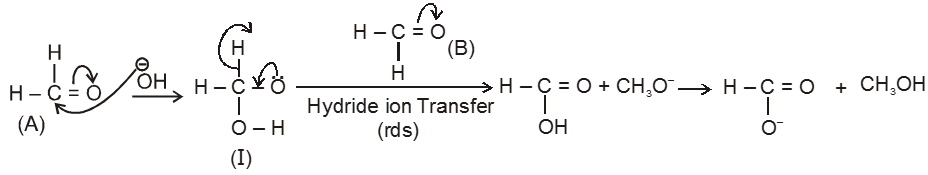
By this mechanism it is clear that acid corresponds to that carbonyl compound over which ![]() can attack easily as nucleophile.
can attack easily as nucleophile.
Note : It is observed that hydride ion transfer from (I) to Carbonyl compound (B) is rate determining step.
Crossed Cannizzaro reaction :
On using two types of carbonyl compounds not having a-hydrogen atom, acid salt will be corresponding to that aldehyde over which ![]() will approach without any hindrence.
will approach without any hindrence.
(i) 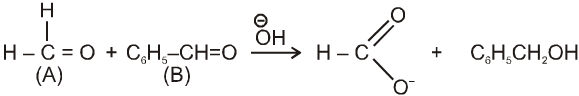
(ii) 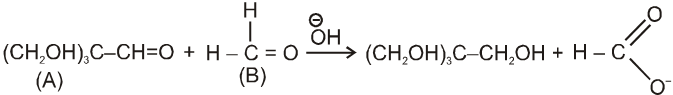
in case (i) ![]() will easily go to (A) and in case (ii) it will go to (B) hence acid salt will be formate ion in both the cases.
will easily go to (A) and in case (ii) it will go to (B) hence acid salt will be formate ion in both the cases.
Intramolecular Cannizzaro reacion :
Here two carbonyl groups (without a-hydrogen atom) are present within the same molecule.
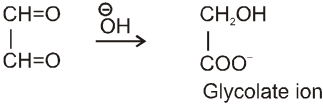
Mechanism :
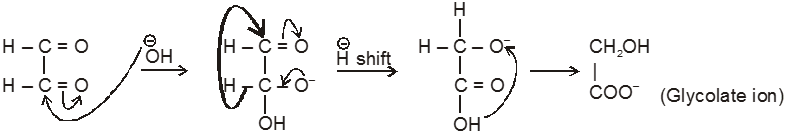
Ex.
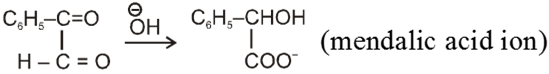
Wittig reaction :
It is used to get alkene from carbonyl compound using phosphorus ylide via the formation of cyclic structure betaine.
Mechanism :
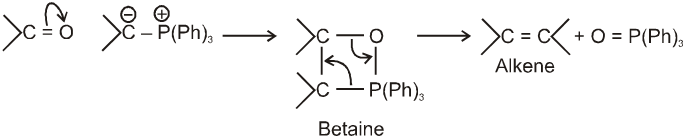
Note : Phosphorus ylides are prepared from alkylhalide and triphenylphosphine in the presence of base like sodium ethoxide as –
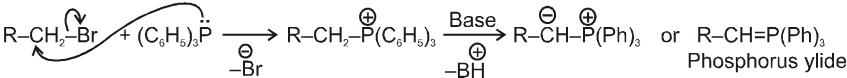
Ex.
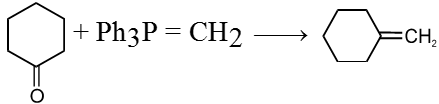
Reduction reactions
(I) Reduction to alcohols :
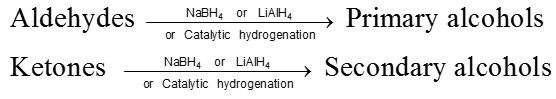
(II) Clemmensen reduction :
Used to get alkane from carbonyl compounds.
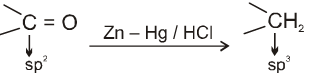
Note : Generally this reaction is avoid if acid sensitive groups are present in the carbonyl compounds.
(III) Wolf-Kishner reduction :
Used to get alkane from carbonyl compounds.
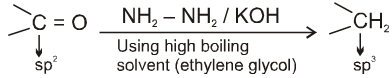
Note : Generally this reaction is avoid if base sensitive groups are present in the carbonyl compounds
(IV) Reaction with PCl5 :
Carbonyl compounds give gemdihalides
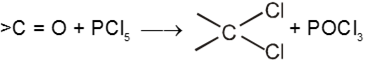
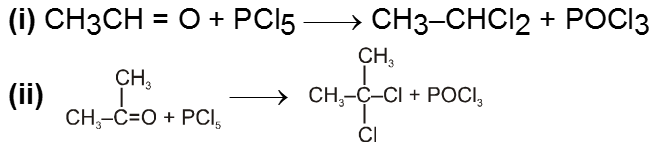
(V) Pinacol-Pinacolone rearrangement :
Pinacole is obtained when 2 moles of acetone are heated with divalent active metal magnesium followed by treating with water.
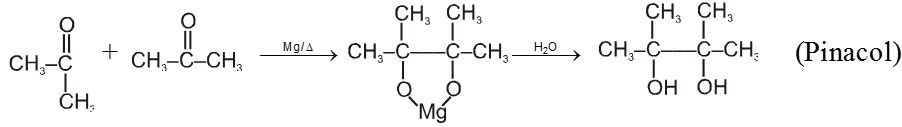
Pinacole undergoes rearrangement in acidic media to give pinacolone
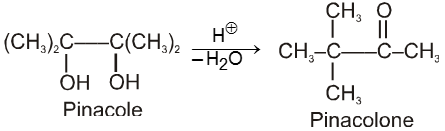
Oxidation reactions
(I) Haloform reaction :
Acetaldehyde and methylalkyl ketones react rapidly with halogen (Cl2, Br2 or I2) in the presence of alkali to give haloform and acid salt.
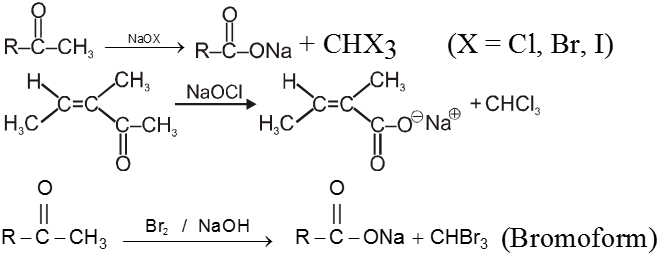
In this reaction – CH3 of 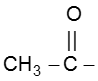 group is converted into haloform as it contains acidic hydrogen atom and rest-part of alkyl methyl ketone give acid salt having carbon atom corresponding to alkyl ketone.
group is converted into haloform as it contains acidic hydrogen atom and rest-part of alkyl methyl ketone give acid salt having carbon atom corresponding to alkyl ketone.
Preparation of haloform from methylketone involves two steps.
(a) Halogenation
(b) Alkalihydrolysis
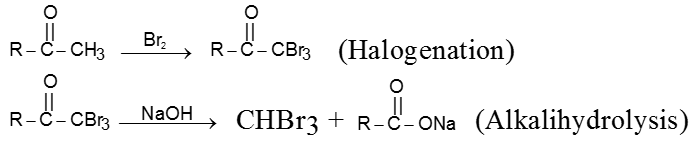
Note : This reaction is used to distinguish the presence of 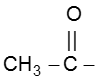 group.
group.
(II) Oxidation of Aldehydes :
• Aldehydes are oxidised to carboxylic acids by common oxidising agents such as KMnO4, HNO3, K2Cr2O7, etc.
![]()
• Aldehydes are also oxidised by mild oxidising agents such as Tollen’s reagent and Fehling’s reagent. On the other hand, ketones are not oxidised by mild oxidising agents.
• Ketones are oxidised under vigorous conditions, i.e., by strong oxidising agents and at elevated temperatures.
It involves carbon-carbon bond cleavage.
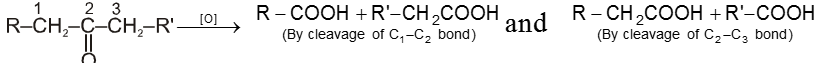
(a) Tollen’s reagent :
It is ammonical silver nitrate solution, prepared by adding ammonium hydroxide to AgNO3 solution. During reaction, first Ag2O is formed which is dissolved in ammoniumhydroxide to give Tollen’s reagent.
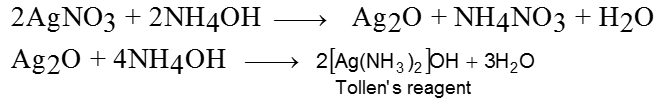
Tollen’s reagent is weak oxidising agent. It gives Ag mirror test with aldehyde.

(b) Fehling’s solution :
It is an alkaline solution of cupric ion complexed with sodium potassium tartarate.
There are two solutions in Fehling solution
Solution (A) CuSO4 solution and
Solution (B) Alkaline solution of sodium potassiumtartarate.
When these two solutions are mixed we get deep blue coloured solution.
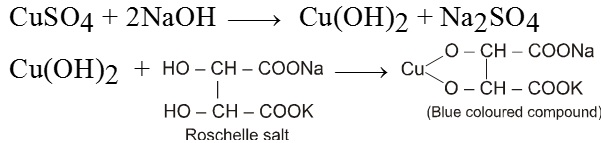
Equal volume of both the solutions are heated with aldehyde to give red brown precipitate of cuprous oxide (Cu2O) which confirms the presence of aldehyde
![]()
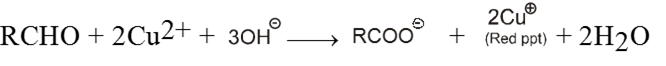
(c) Benedict solution :
It also consists of two solutions.
Solution (A) CuSO4 solution and
Solution (B) Alkaline solution of sodium Citrate.
CuSO4 + 2NaOH Cu(OH)2 + Na2SO4
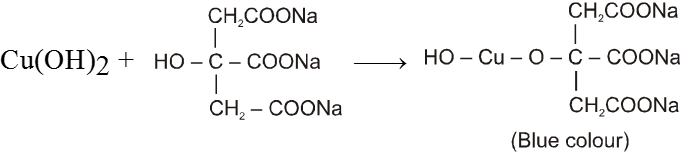
Aldehyde gives positive test with Benedict solution.
![]()
(d) Schiff’s reagent :
It is dilute solution of rosaniline hydrochloride whose pink colour has been discharged by passing SO2. Aldehyde restores pink colour when treated with schiff’s reagent (Magenta solution in H2SO3).
Other miscellaneous reactions :
(I) 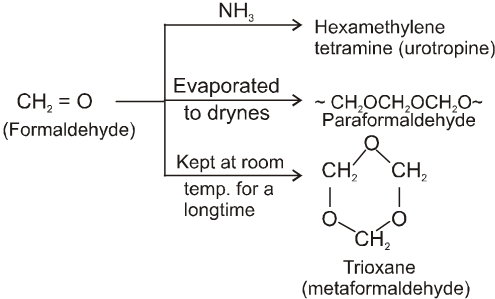
(II) 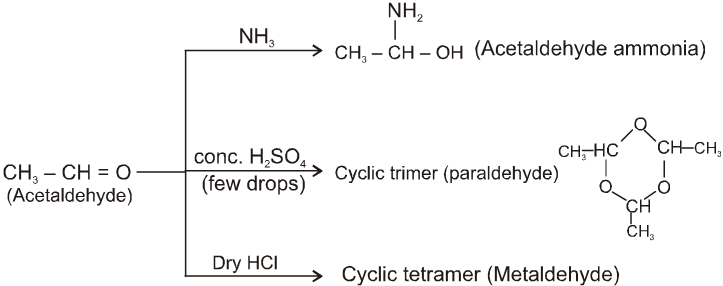
(III) 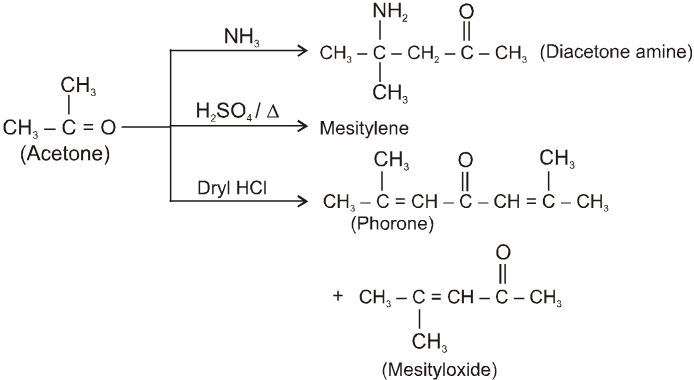
(IV) 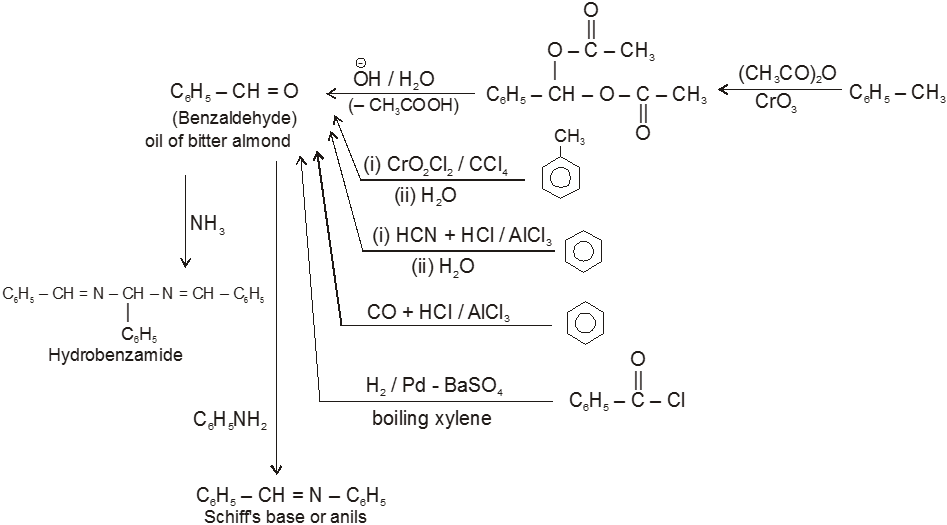
(V) 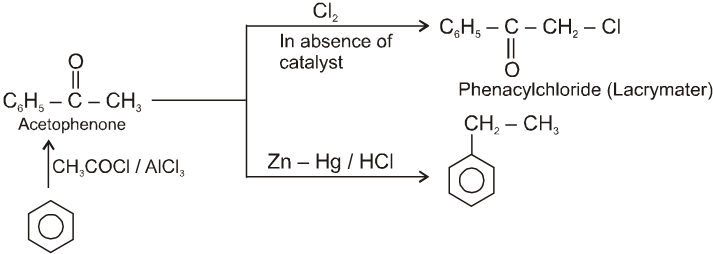

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
