- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
MATHEMATICAL ANALYSIS OF CUBIC SYSTEM
(TYPES AND ANALYSIS)
Simplest crystal is to be studied in cubic system. Three types of cubic systems are following.
(a) Simple Cubic (SC) : Atoms are arranged at the corners of the cube.
(b) Body Centered Cubic (BCC) : Atoms are arranged at the corners and at the centre of the cube.
(c) Face Centered Cubic (FCC) : Atoms are arranged at the corners and at centered of the each faces.
Contribution of different Lattice point in one Cubical unit cell :
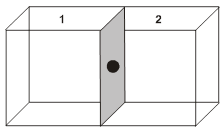
(i) Contribution from one corner lattice point =![]() th.
th.
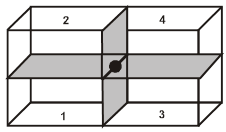
(ii) Contribution from one face centered lattice point =![]() .
.
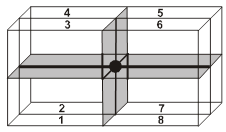
(iii) Contribution from edge centered lattice point = ![]() th.
th.
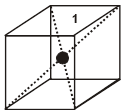
(iv) Contribution from body centered lattice point = 1.
Number of atoms per unit cell / unit cell contents :
The total number of atoms contained with in the unit cell for a simple cubic called the unit cell content.
(a) Simple cubic structure (sc) :
Each corner atom is shared by eight surrounding cubes. Therefore, it contributes for ![]() of an atom.
of an atom.
![]()
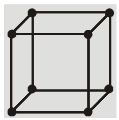
(b) Body centered cubic structure (bcc) :
(i) Eight Corner atoms contribute one atom per unit cell.
(ii) Centre atom contribute one atom per unit cell.
(iii) So, total 1 + 1 = 2 atoms per unit cell.
![]()
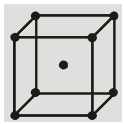
(c) Face centered cubic structure (fcc) :
(i) The eight corners atoms contribute for ![]() of an atom and thus one atom per unit cell.
of an atom and thus one atom per unit cell.
(ii) Each of six face centered atoms is shared by two adjacent unit cells and therefore one face centred atom contribute half of its share. Means
![]() atom per unit cell.
atom per unit cell. 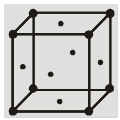
(iii) So, total Z = 3 + 1 = 4 atoms per unit cell.
Atomic radius :
It is defined as the half of the distance between nearest neighbouring atoms in a crystal. It is expressed in terms of length of the edge (a) of the unit cell of the crystal.
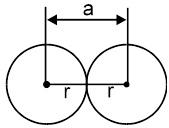
(a) Simple cubic structure [S.C.]
Radius of atom ‘r’ =![]()
(b) Face centered cubic structure (FCC) ‘r’ = ![]()
(c) Body centered cubic structure (BCC) ‘r’ =![]()
a = b = c
a = b = g = 90º

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
