- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
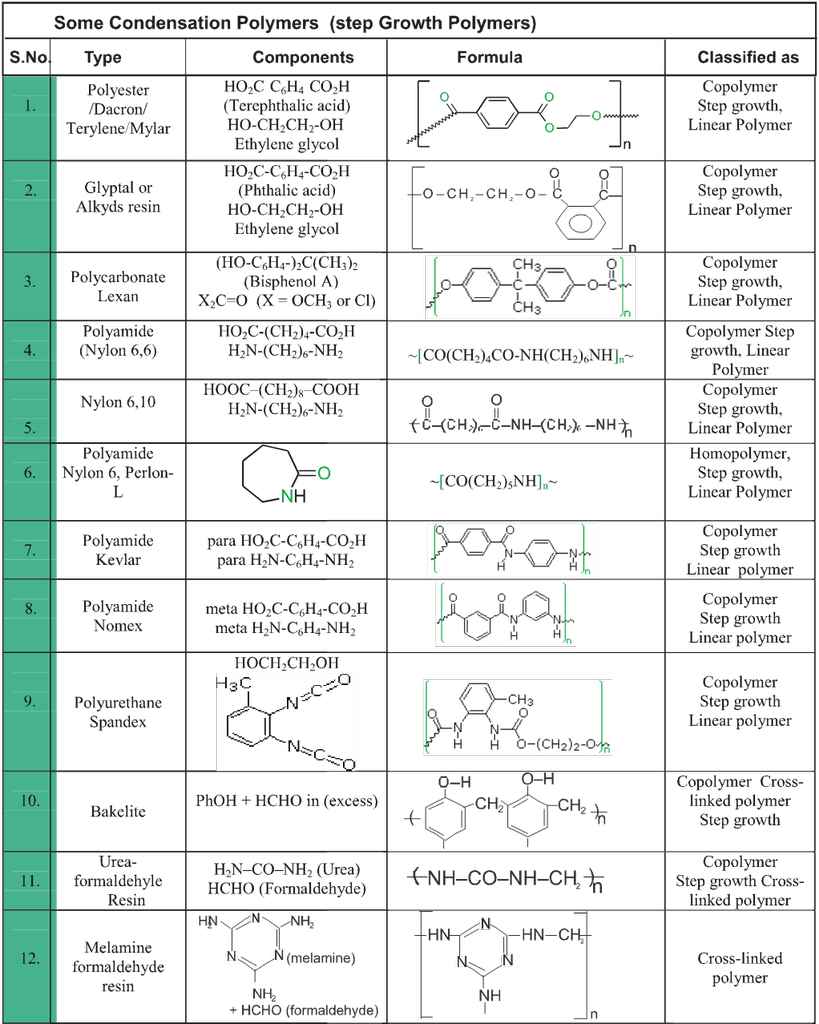
Classification- Polymers can be classified by following ways
(A) Classification based on origin or source :
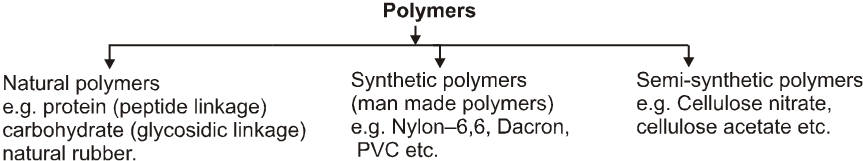
(B) Classification based on synthesis :

(C) Classification based on Structure:
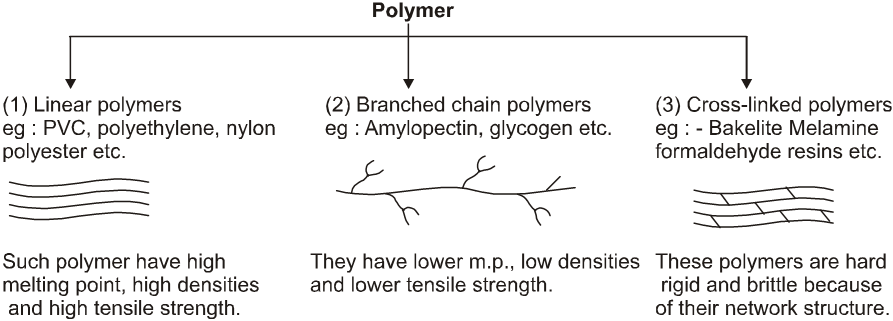
(D) Classification based on Mechanism :
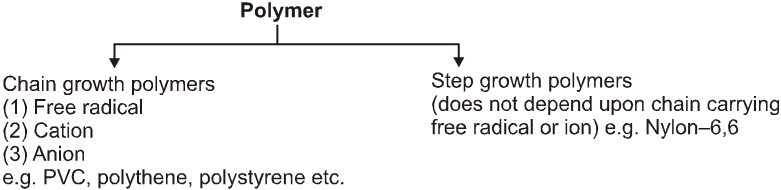
(1) Free radical :
This mechanism involve catalyst which generate free radical. Steps involved are :

(E) Calssification based upon molecular force :
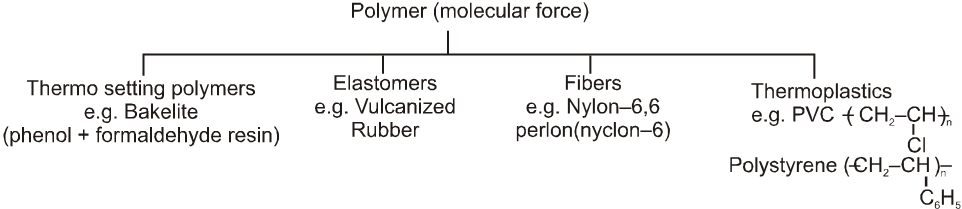
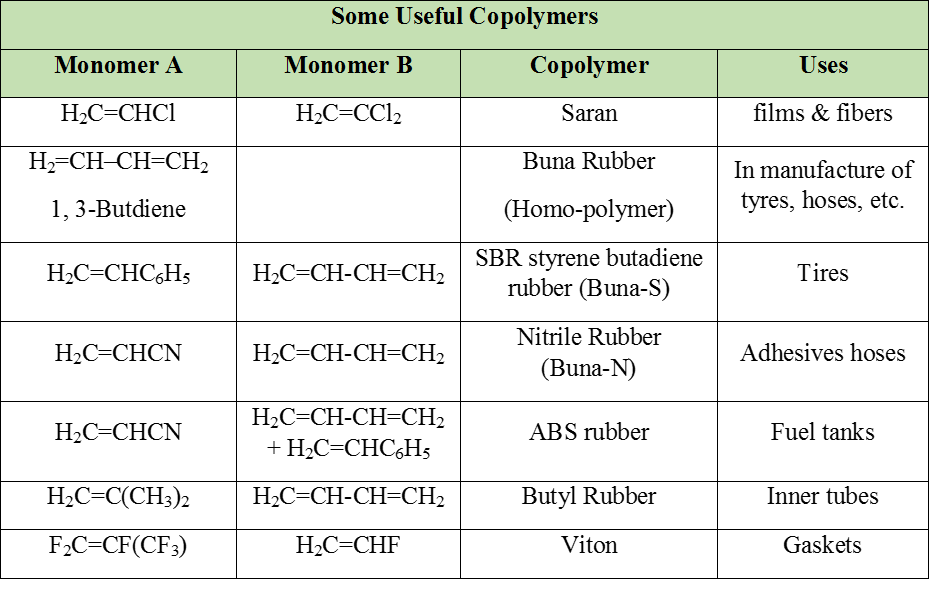
(i) Elastomers : Polymers in which the intermolecular forces of attraction between the polymer chains are the weakest are called elastomers.
e.g. Buna rubber, Natural rubber etc.
(ii) Fibres : Polymers in which the intermolecular forces of attraction are the strongest are called fibers. These forces are either due to H-bonding or dipole-dipole interactions. In case of nylon (polyamides), the intermolecular forces are due to H-bonding while in polyesters (terylene, dacron) and polyacrylonitrile (orlon, acrylin) Dipole-dipole interactions between the polar carbonyl (C = O) groups and, between carbonyl and cyano (– C º N) groups respectively.
(iii) Thermoplastics : Polymers in which the intermolecular forces of attraction are in between those of elastomers and fibres are called thermoplastics. The process of heat softening and cooling can be repeated as many times as desired without any change in chemical composition and mechanical properties of the plastic.
e.g. Polyethylene, polypropylene, polystyrene, PVC, teflon etc.
(iv) Thermosetting polymers : These are semifluid substances with low molecular weights which when heated in a mould, undergo change in chemical composition to give a hard, infusible and insoluble mass. This hardening on heating is due to extensive cross-linking between different polymer chains to give a three-dimensional network solid. Examples : Phenol-formaldehyde (bakelite), urea-formaldehyde, etc.
e.g. Bakelite urea-formaldehyde resin, terylene etc.

Natural rubber :
Natural rubber is a polymer of isoprene, and obtained from natural source-latex tree. In natural rubber, isoprene units are joined together in head-to-tail fashion and all double bonds in the polymer chain have cis configurations as shown in the given figure.
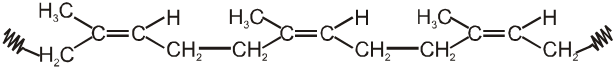
(Natural rubber)
The polymer contains cis repeating units and has a molecular weight ranging from 100,000 upto 1,000,000. A related polymer, called gutta percha which has a structure with trans double bonds and a much lower molecular weight. A typical sample of gutta percha has a molecular weight of about 7,000.
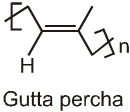
The cis arrangement of the double bonds in natural rubber prevents the rubber molecules from fitting into an ordered structure. Thus, rubber is an amorphous polymer. Because of the random coiling of its polymer chains, rubber stretches easily. When strectched, the rubber molecules are forced into a higher energy state. When the tension is released, rubber snaps back to its original random coiled state.
Vulcanization of Natural Rubber
Raw rubber is soft and tacky, they have very low mechanical strength, affected by environmental factors such as light, temperature, and oxygen and are of no use for any commercial purpose. These factors make rubber unsuitable for a number of applications. To enhance the mechanical properties and stiffness of natural rubber in 1839, charles Goodyear devised a method of reacting rubber with sulphur to form a more durable material.
Valcanization is a process in which natural rubber is heated with sulphur, where it undergo crosslinking and connect the isolated rubber chain through a network making them strong and stiff. Vulcanization forms both the cyclic structure shown on the left and the more desirable cross-linked structure shown on the right in the following figure.
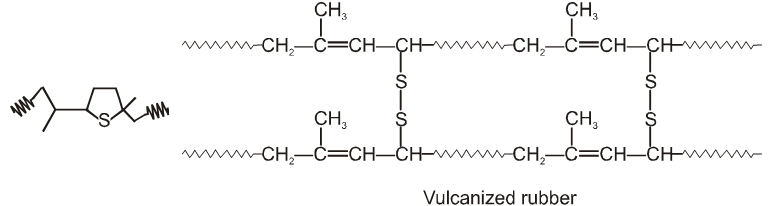
Increasing the amount of sulphur makes the vulcanized polymer harder and more durable. Adding 3-5% sulphur makes a product good for rubber bands and inner tubes. Adding 20-30% sulphur makes a hard rubber that was once widely used in ways that a hard synthetic plastic is used today. The vulcanization process made early automobile tires possible.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
