- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Methods to monitor the progress of the reaction :
(A) Pressure measurement :
Progress of gaseous reaction can be monitored by measuring total pressure at a fixed volume & temperature.
This method can applied for those reaction also in which a gas is produced because of decomposition of a solid or liquid. We can get an idea about the concentration of reacting species at a particular time by measuring pressure.
The pressure data can be given in terms of
(i) Partial pressure of the reactant
(ii) Total pressure of the reaction system
(iii) Pressure at only some points of time
(b) Volume measurement :
(i) By measuring the volume of product formed we can monitor the progress of reactions.
(c) Optical rotation measurment :
It is used for optically active sample. It is applicable if there is atleast one optically active species involved in chemical reaction.
The optically active species may be present in reactant or product.
It is found that 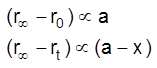
(a = concentration , x = amount consumed)
where arer0, rt, r¥ are angle of optical rotation at time t = 0, t = t and t = ¥

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
