- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Methods used for the preparation of Aldehydes only
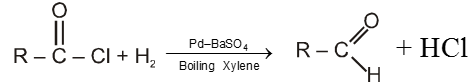
Rosenmund's reduction :
Here acid chlorides are reduced to aldehyde with H2 in boiling xylene using palladium as a catalyst supported on barium sulphate.
Note :
(a) Pd Catalyst is poisoned by BaSO4 to check further reduction of aldehyde to alcohol.
(b) Formaldehyde cannot be obtained by this method because HCOCl is unstable at common temperature.
(c) Reaction with acid chloride and dialkyl cadmium we can obtain ketone.
Stephen's reduction :
![]()
Oxo-process :
It is also called as carbonylation here alkene reacts with water gas at high temperature and pressure in the presence of cobalt carbonyl catalyst to give aldehyde.
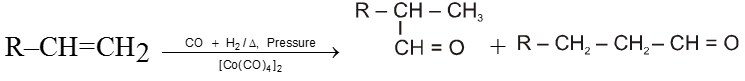
Reimer-Teimann Reaction :
By this method phenolic aldehyde is prepared

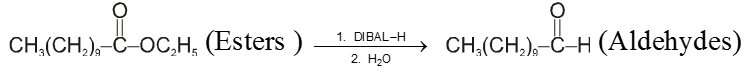
From esters or nitrile :
![]()
DIBAL–H : Diisobutyl aluminium hydride [AlH(i-Bu)2] is a reducing agent.
From hydrocarbons :
By oxidation of methyl benzene and its derivative using chromyl chloride (CrO2Cl2)
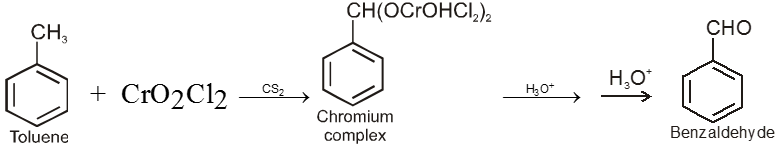
This reaction is called Etard reaction.
By oxidation of methyl benzene and its derivative using chromic oxide (CrO3) in acetic anhydride:

By Gatterman-Koch reaction :
![]()
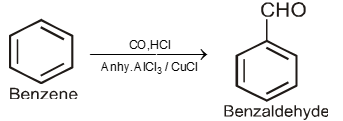

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
