- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Integrated rate laws
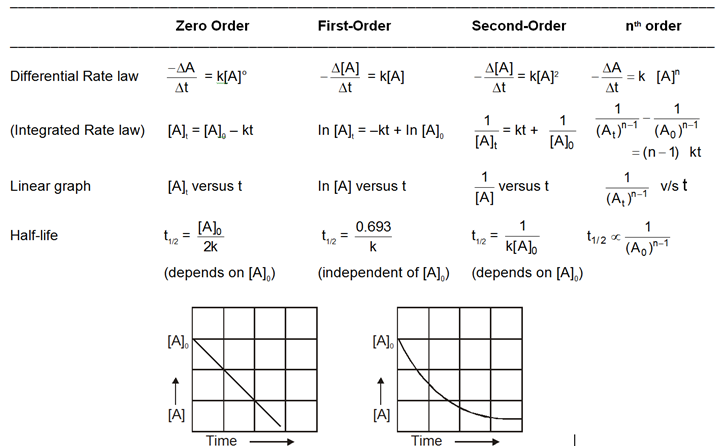
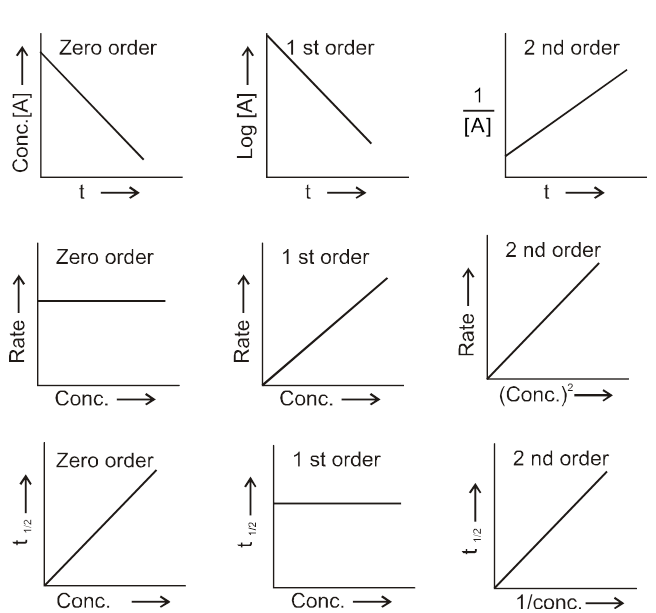
(a) Zero Order Reactions :
For a zero order reaction
General rate law is, Rate = k [conc.]º = constant
If C0 is the initial concentration of a reactant and Ct is the
concentration at time ‘t’ then
Rate = k = ![]() or kt = C0 – Ct or Ct = C0 – kt
or kt = C0 – Ct or Ct = C0 – kt
Unit of K = same as that of Rate = mol lit–1 sec–1.
Time for completion =![]()
t1/2 (half life period) at t1/2 , Ct =![]() , so kt1/2 =
, so kt1/2 = ![]() Þ t1/2 =
Þ t1/2 = ![]()
t1/2 µ C0
Examples of zero order reactions :
Generally decomposition of gases on metal surfaces at high concentrations follow zero order kinetics.
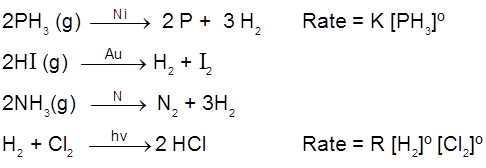
(b) First Order Reactions :
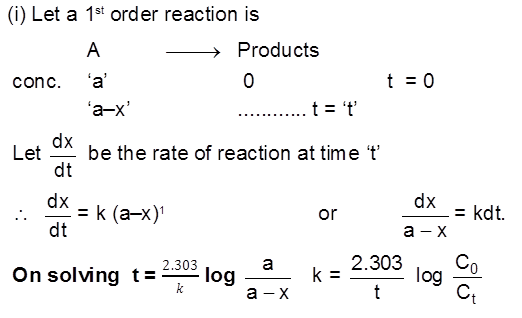
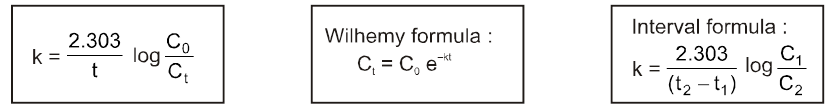
If any substance is growing/increasing following first order kinetics then :
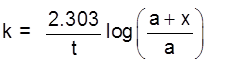
where a is initial concentration of the substance and x is the increment in its concentration after time t.
Half life time (t1/2)
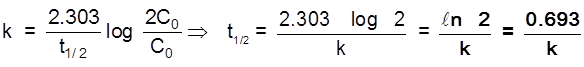
Half life period for a 1st order reaction is a constant quantity.
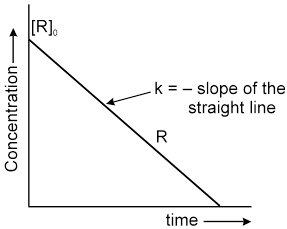
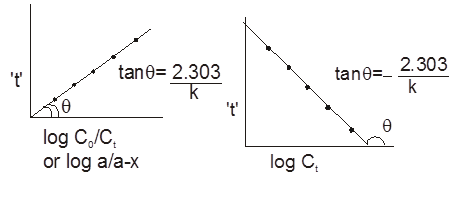
Graphical Representation :
![]()
First order growth reaction :
For bacteria multiplication or virus growth use following concept Consider a growth reaction
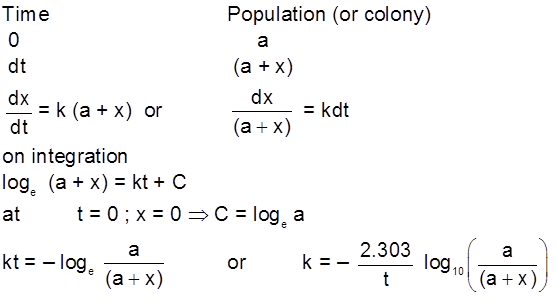
or
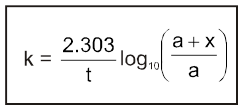
Generation time :
At ¾® t = generation time , x = a
t = ![]()
Examples of 1st order reactions :
1. H2O2 ¾® H2O + ![]() O2
O2
2. NH4 NO2 ¾® 2H2O + N2
3. Radiactive decay
All radioactive decays are always first order kinetics.
![]()
(c) Second order reaction :
2nd order Reactions
Two types
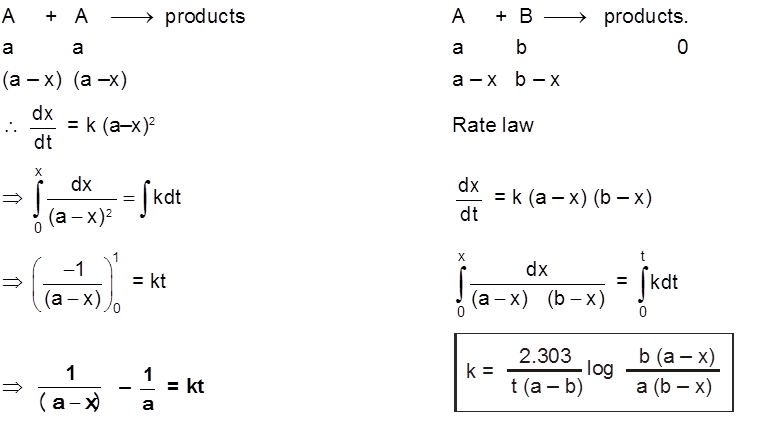
(d) Psuedo first order reaction :
A second order (or of higher order) reactions can be converted into a first order reaction if the other reactant is taken in large excess. Such first order reactions are known as psuedo first order reactions.
For A + B ¾® Products [Rate = K [A]1 [B]1]

Now if ‘B’ is taken in large excess b > > a.
![]()
‘b’ is very large can be taken as constant
Þ kb = log Þ k¢ = log
k¢ is psuedo first order rate constant
K’ will have units of first order.
K will have units of second order.
Examples of Pseudo 1st order reactions :
(a) Hydrolysis of canesugar
C12H12O11 + H2O ¾¾® C6H12O6 + C6H12O6
sucrose excess
(b) Hydrolysis of esters
CH3COOCH3 + H2O ![]() CH3COOH + CH3OH
CH3COOH + CH3OH
(excess)
Table : Characterstics of First-and Second-Order Reactions of the Type A Products
Graphical comparison of different orders
(A) integrated rate law method :
It is method of hit and trial. By checking where the kinetic data (experimetal data) best fits into which integrated rate law , we determine the order. It can also be done graphically.
(B) Method of half lives :
The half lives of each order is unique so by comparing half lives we can determine order
(C) Ostwald’s isolation method :
This method is useful for reaction which involve a large number of reactants. In this method, the concentration of all the reactants are taken in large excess exception that of one, so if
rate = k [A]a [B]b [C]c = k0 [A]a
Then value of ‘a’ can be calculated by previous methods and similarly ‘b’ and ‘c’ can also be calculated

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
