- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Purification or Refining of Metal
Physical Method :
(1) Liquiation Process :
Based on difference in melting point of metal and impurity. This process is used for purification of those metal which have low melting point than each of impurities associated with metal.
Used for Purification of
(i) Impure tin metal (ii) Impure zinc (spelter)
Spelter Þ 97 % to 98 % zinc is known as spelter.
(iii) For removing Pb from Zn – Ag alloy.
(2) Fractional Distillation process
Used for purifying those metal which are easily volatile, while impurities present in it are not used for purifying Zn , Cd & Hg.
(3) Zone refining of metal
Principle : Based on principle that an impure molten metal on gradually celling will deposit crystal of pure metal while the impurity will be left in the remaining part of molten metal.
*This method is used when metal is required in very high purity.
Mainly used for purification of Si & Ge.
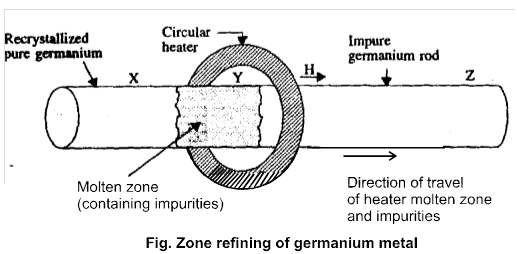
Note : High purity of metal can be obtained by zone refing method , if impurities have lower melting point.
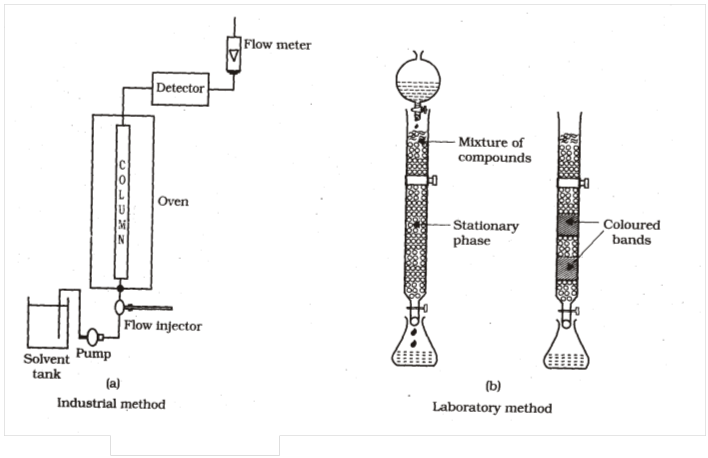
(4) Chromatographic method
Principle : Based on the principle of adsorption.
*Different component of a mixture are differently adsorbed on an adsorbent.
Chemical Method
(1) Oxidative Refining
Used when impurities present in metal are easily oxidised by oxygen.
Mainly used for refining Þ Pb , Ag , Cu , Fe .....
In this method , molten impure metal is subjected to oxidation.
(i) Bessemerisation [Purification of Iron]
Mainly used for manufacture of steel from cast iron.
Haematite ore  pig iron
pig iron  steel
steel
P is also oxidise to P4O10
P4 (l) + 5O2 ® P4O10 (l)
6 CaO (g) + P4O10 (l) ® 2 Ca3 (PO4)2 (l)
Thomas Slag
Which is used as fertilizer.
(ii) Cupellation (Removal of lead)
Mainly used to remove Pb from Ag or Au.
(2) Poling Process
This method is used for purification of those impure metal which contain their own oxide as one of the impurities.
Eg. (a) Purification of Cu.(b) Purification of impure Tin.
(3) Electrolytic refining
Metal , Cu , Ni & Al refined electrolytically.
The anode mud obtained in the electrolytic refining has
Electrolytic refining Anode Mud
(i) Lead Ag , Au , Sb , Cu.
(ii) Cu Ag , Au , Pt.
(iii) Ag Au , Pt.
(4) (a) Kroll's Process Þ For Ti & Zr.
TiCl4 + 2 Mg ![]() Ti + 2MgCl2
Ti + 2MgCl2
(b) IMI process Þ [ Imperial Metal industries ]
TiCl4 + 4 Na  Ti + 4 NaCl.
Ti + 4 NaCl.
Vapour phase refining :
(a) Extraction of Nickel [ Mond's Process ]
Used for purification (refining) of Ni.
Ni form complex with Co , [Ni(CO)4] which volatilized
[BP=43°] & then decompose at 200°C in Ni & CO.
Ni(S) + 4 CO ![]() Ni (CO)4(g)
Ni (CO)4(g) ![]() Ni + 4 CO.
Ni + 4 CO.
readily volatile
(B.P. = 43°C)
(b) VanArkel – DeBoer Process
Used for purification of Ti , Zr , Bi & B.
Small amount of puremetal is obtained.
Impure Ti(S) + 2I2 ![]() TiI4(g)
TiI4(g) ![]() Ti + 2I2
Ti + 2I2
KROLL’S PROCESS :
TiCI4 + 2 Mg ![]() Ti + 2 MgCI2 (Kroll’s process)
Ti + 2 MgCI2 (Kroll’s process)
TiCI4 + 4 Na  Ti + 4 NaCI (Imperial metal industries (IMI) process)
Ti + 4 NaCI (Imperial metal industries (IMI) process)
NaCI is leached with H2O. Ti is in the form of small granules. These can be fabricated into metal parts using “powder forming” techniques and sintering in an inert atmosphere. Zr is also produced by Kroll’s process.
VAPOR PHASE REFINING :
(i) Extraction of Nickel (Mond’s process) :
Nickel is extracted from sulfide ore by roasting followed by reduction with carbon, but the process is complicated by the fact that nickel is found in association with other metals. The refining is rather unusual, for nickel forms a complex with carbon monoxide tetracarbonylnickel (O) [Ni(CO)4]. This substance is molecular in molecular in structure and readily volatilized (boiling point 43ºC). It is made by heating nickel powder to 50ºC, in a stream of CO and then decomposed at 200ºC. Any impurity in the nickel sample remains in the solid state and the gas is heated to 230ºC, when it decomposes, giving pure metal and CO, which is recycled. Ni(CO)4 is gaseous and may be produced by warming nickel with CO at 50ºC.
The sequence of reaction is
H2O(g) + C ® CO(g) + H2
Ni(s) + 4 CO(s) ![]() [Ni(CO4)] (g)
[Ni(CO4)] (g)
[Ni (CO)4](g) ![]() Ni + 4CO(g)
Ni + 4CO(g)
(ii) Van Arkel–De Boer process :
Small amounts of very pure metals (Ti, Zr, or Bi) can be produced by this method. This process is based on the fact that iodides are the least stable of the halides. The impure element is heated with iodine, producing a volatile iodide, TiI4, ZrI4, or BiI3. These are decomposed by passing the gas over an electrically heated filament of tungsten or tantalum that is white hot. The element is deposited on the filament and the iodine is recycled. As more metal is deposited on the filament, it conducts electricity better. Thus, more electric current must be passed to keep it white hot. Thus the filament grows fatter and eventually the metal is recovered. The tungsten core is distilled out of the center and a small amount of high purity metal is obtained.
Impure ![]()
The method is very expensive and is employed for the preparation of very pure metal for specific use.
(iii) Parke’s process :
The removal of the impurities of Ag from the commercial lead is called desilverisation of lead and is done by Parke’s process . Thus, Parke’s process is the desilverisation of lead.
In Parke’s process, the commercial lead, which contains Ag as impurities, is melted in iron pots and 1% of Zn is added to it. The molten mass is thoroughly agitated. Since Ag is about 300 times more soluble in Zn than in Pb, most of the Ag present in the commercial lead as impurity mixes with Zn, to form Zn–Ag alloy. When the whole is cooled, two layers are obtained. The upper layer contains Zn–Ag alloy in the solid state, while the lower layer has lead in the molten state. This lead contains only 0.0004% of Ag and hence is almost pure. Lead obtained after removing most of Ag from it (desilverisation of lead) by Parke’s process, is called desilverised lead. This lead contains the impurities of metals like Zn, Au, Sb etc. These metal impurities are removed from desilverised lead by Bett’s electrolytic process.
Zn–Ag alloy, formed in the upper layer, is skimmed off from the surface of the molten lead by perforate ladles. This alloy contains lead as impurity. This impurity of Pb is removed from the alloy by liquation process, in which Zn–Ag alloy is heated in a slopping furnace, when the impurity of Pb melts and hence drains away from the solid alloy. Thus purified Zn–Ag is obtained. Now Ag can be obtained from this purified Zn–Ag alloy by distillation process, in which the alloy is heated strongly in presence of little carbon in a fire–clay retort. Zn, being more volatile, distills off while Ag remains in the retort, carbon used in the process reuses the oxide of Zn, if formed. Ag obtained from Zn–Ag alloy is contaminated with a little of Pb as impurity. This impurity of Pb placed in a cupel (cupel is a boat–shaped) dish made of bone ash which is porous in nature) in a reverberatory furnace and heated in the presence of air. By doing so, lead (impurity) is oxidised to PbO(litharge) which volatilises and pure Ag is left behind in the cupel. Last traces of PbO are absorbed by the porous mass of the cupel.
(iv) Pudding process : This process is used for the manufacture of wrought iron from cast iron. We know that cast iron contains the impurities of C, S, Si, Mn and P. When these impurities are removed from cast iron, we get wrought iron. In this process the impurities are oxidised to theiro xides not by blast of air but by the haematite (Fe2O3) lining of the furnace.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
