- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Expressing concentration of solutions
Concentration Terms :
% Concentration
Mass percentage. :It is the amount of solute in grams dissolved per 100 g of solution. e.g., 10% solution of sodium chloride means 10 g of solid sodium chloride present in 100 g of solution
% w/w = ![]() × 100
× 100
Ex. 10% w/w urea solution = 10 g of urea is present in 100 g of solution.
= 10 g of urea is present in 90 g of water.
Mass by volume percentage (% w/v) : It is defined as mass of solute dissolved per 100 ml of solution. It is commonly used in medicine and pharmacy.
% wt/vol. (w/v)
% w/v = wt. of solute/100 mL of solution
% w/v = 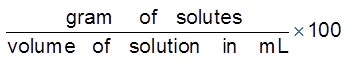
Ex.
10% (w/v) urea solution. = 10 g of urea is present in 100 mL of solution.
But not 10 g of urea present in 90 ml of water for dilute solution : volume solution = volume solvent.
Volume percentage (% v/v) : It is defined as volume of a solute dissolved per 100 ml of solution.
% v/v = ![]() × 100
× 100
Strength of solution in g/L : Weight of solute (in gram) per litre (1000 mL) of solution.
Ex.
10% (w/v) sucrose solution, then specify its concentration in g/L
100 mL .......... 10 g
1000 mL .......![]() = 100 g/L
= 100 g/L
Molarity (M) : It is expressed as the number of moles of solute per litre of solution.
Molarity = No. of moles of solute per litre of solution.
Let n = No. of moles of solute ; N = No. of moles of solvent ; V = volume of solution
![]()
no. of moles of solute = molarity x volume ( in L)
no. of m. moles of solute = molarity x volume ( in mL)
If V1 mL of C1 molarity solution is mixed with V2 mL of C2 molarity solution (same substance or solute)
Cf (V1+V2) = C1V1 + C2V2
Cf = 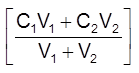 =
= ![]() where Cf = molarity of final solution
where Cf = molarity of final solution
Molality (m) : It is defined as number of moles of solute per 1000 g or 1 kg of solvent.
Molality = No. of moles of solute per kg(1000 g) of solvent.
Let w gram of solute (Molar mass = Mg/mole) is dissolved in 'W' gram of solvent.
molality = ![]() molality =
molality = ![]()
Molality not depends on temperature.
Normality : It is defined as number of gram equivalents of solute dissolved per litre of solution.
No. of equivalents per litre of solution = 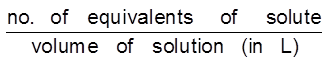 = n-factor molarity
= n-factor molarity
No. of equivalents = normality × volume (in L)
Equivalent mass = 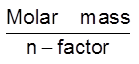
No. of equivalent=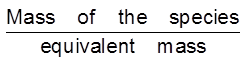 =
= 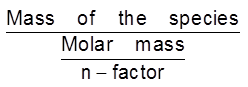
'n' - factor
(i) For oxidizing/reducing agents : no. of e– involved in oxidation/reduction half reaction per mole of oxidising agent /reducing agent.
e.g. : 5e– + 8H+ + MnO4– =Mn2+ + H2O n- factor = 5
(ii) For acid/ base reactions : no. of H+ ions displaced/ OH– ions displaced per mole of acid/ base.
e.g. : NaOH n - factor = 1 H2SO4 n - factor = 2
(iii) For salt : n = Total charge on cations.
or ![]()
total charge on anions
e.g. : Al2(SO4)3 n - factor = charge on the cation = 2 x 3 = 6
Mole-fraction (x) : It is the ratio of number of moles of a particular component to the total number of moles of all the components. e.g., mole-fraction of component A, xA = 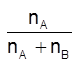 , where nA is the number of moles of component 'A' and nB is the number of moles of component 'B'.
, where nA is the number of moles of component 'A' and nB is the number of moles of component 'B'.
For binary mixture.
Xsolute = ![]() =
= ![]() ; XSolvent =
; XSolvent = ![]() =
= ![]()
Xsolute + XSolvent = 1
Parts per million (ppm) : The number of parts of solute present in 1 million parts of solution are called its ppm. When a solute is present in small quantities (very minute amounts), it is easier to express the concentration in parts per million.
(a) ppm (w/w) = 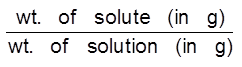 × 106
× 106
(b) ppm (w/v) = 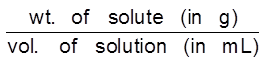 × 106
× 106
(c) ppm (moles/moles) = 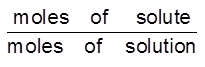 × 106
× 106
Table : 1

Note : All volume related concentration terms are temperature dependent.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
