- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
OZONE (O3)
O3 is an allotropic form of oxygen. At a height of about 20 Kms it is formed from atmoshperic oxygen in the presence of sunlight. This O3 layer protects the earth’s surface from an excessive concentration of ultra violet radiations.
Preparation :
It is prepared by passing silent electric discharge through a slow stream of pure and dry oxygen to prevent its decomposition.
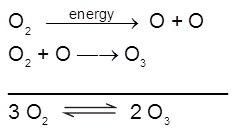
DHV (298 K) = + 142 kJ mol–1
The product is known as ozonised oxygen. If concentration of O3 greater than 10% are required , a battery of ozonisers can be used , and pure ozone (bp 385 K) can be condensed in a vessel surrounded by liquid oxygen.
Properties :
Physical properties :
(1) It is a pale blue gas which forms a blue liquid and one solidification forms violet black crystals.
(2) It has a strong fish – like smell
(3) It is slightly soluble in water but more in turpentine oil or glacial acetic acid or CCl4 .
(4) O3 molecule is diamagnetic but O3– ion is paramagnetic (1 unpaired e–)
(5) It is explosive and unstable with respect to O2 as its decomposition into O2 results in the liberation of heats and an increase in entropy.
Chemical Properties :
(1) As Oxidising agent : Due to the ease with which it liberates atoms of nascent oxygen
(O3 —® O2 + O ), it acts as a powerful oxidising agent.
In acidic medium :
O3 + 2 H+ + 2e– —® O2 + 2 H2O SRP = + 2.07 V.
In alkaline medium :
O3 + H2O + 2e– —® O2 + 2 OH– SRP = + 1..24 V
Therefore , Ozone is a stronger oxidising agent in acidic medium.
With excess of potassium iodide solution buffered with a borate buffer, ozone liberates iodine which can be titrated against a standard solution of sodium thiosulphate. This is a quantitative method for estimating O3 gas.
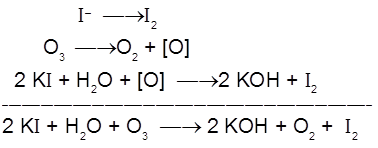
It oxidises PbS to PbSO4 , MnO42– to MnO4– (basic medium) and [Fe(CN)6]4– to [Fe(CN)6]3– (basic medium).
Note : With experimental facts it has been shown that nitrogen oxides (particularly nitric oxide) combine very rapidly with ozone and there is, thus, the possibility that nitrogen oxides emitted from the exhaust systems of supersonic jet aeroplanes might be slowly depleting the concentration of the ozone layer in the upper atmosphere.
NO(g) + O3 (g) —® NO2 (g) + O2 (g)

O–O bond length decreases in order : H2O2 (1.48 Å) > O3 (1.28 Å) > O2F2 (1.22 Å) > O2 (1.21 Å)
Uses :
It is used as a germicide, disinfectant and for sterilising water. It is also used for bleaching oil, ivory, flour starch etc. It acts as an oxidising agent in the manufacture of potassium permanganate

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
