- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Carboxylic Acid Derivatives
Closely related to the carboxylic acids and to each other are a number of chemical families known as functional derivatives of carboxylic acids : acid chlorides, anhydrides, amides, and esters, These derivatives are compounds in which the —OH of a carboxyl group has been replaced by —CI,—OOCR,
—NH2, or —OR`.
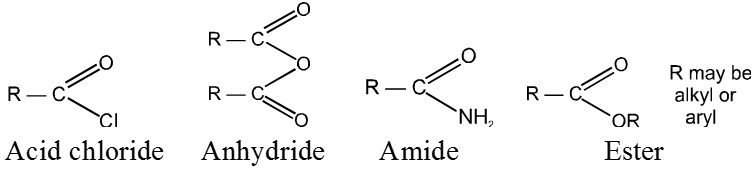
They all contain the acyl group, ![]()
Like the acid to which it is related, an acid derivative may be aliphatic or aromatic, substituted or unsubstituted; whatever the structure of the rest of the molecule, the properties of the functional group remain essentialy the same.
Characteristic reaction of acid deerivatives (Nucleophilic acyl substitution) :
Nucleophilic acyl substitution usually takes place by an addition-elimination mechanism.The incoming nucleophile adds to the carbonyl to form a tetrasubstituted intermediate with a tetrahedral carbon.
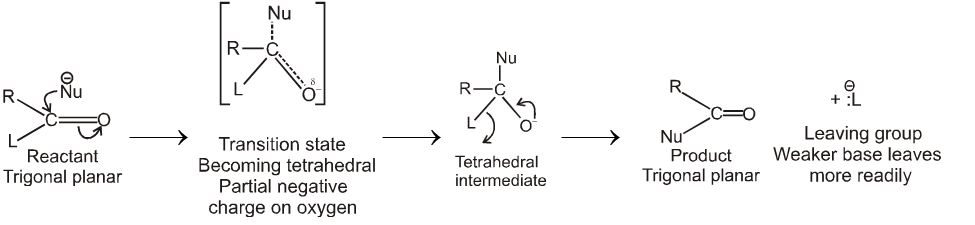
The tetrahedral intermediate formed when a nucleophile attacks the carbonyl carbon of a carboxylic acid derivative is not stable and cannot be isolated.
A pair of nonbonding electrons on the oxygen reforms the bond, and either  or
or  is eliminated with its bonding electrons. Whether
is eliminated with its bonding electrons. Whether  or
or  is eliminated depends on their relative basicities. The weaker base is preferentially eliminated because the weaker the base, the better it is a leaving group.
is eliminated depends on their relative basicities. The weaker base is preferentially eliminated because the weaker the base, the better it is a leaving group.
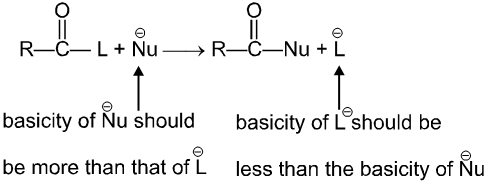
Thus carboxylic acid derivative will undergo a nucleophilic acyl substitution reaction provided that the incoming nucleophile is a stronger base than the group that is to be replaced. If the incoming nucleophile and the group attached to acyl group in the starting material have similar basicities, the tetrahedral intermediate can expect either group with similar ease. A mixture of starting material and substitution product will result.
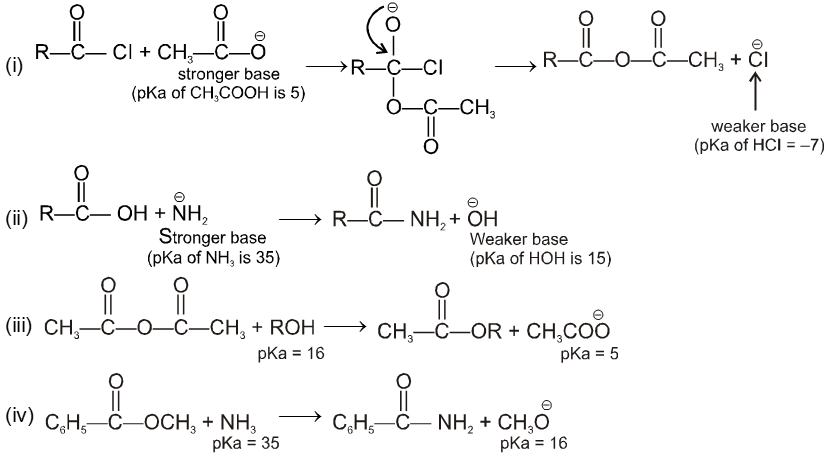
Condition for acyl nucleophilic substitution reaction :
![]()
(i) L must be better leaving group than ![]() , i.e., basicity of Nu should be more than that of
, i.e., basicity of Nu should be more than that of
(ii) ![]() must be a strong enough nucleophilic to attack RCOL.
must be a strong enough nucleophilic to attack RCOL.
(iii) Carbonyl carbon must be enough electrophilic to react with ![]() .
.
Reactivity order : 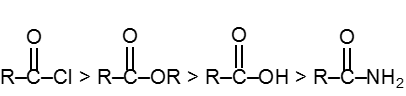
(A) Acid halides :
Methods of preparation of Acyl halides :
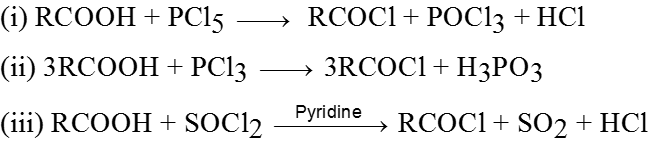
Ex. 
Ex. 
Chemical Reactions :
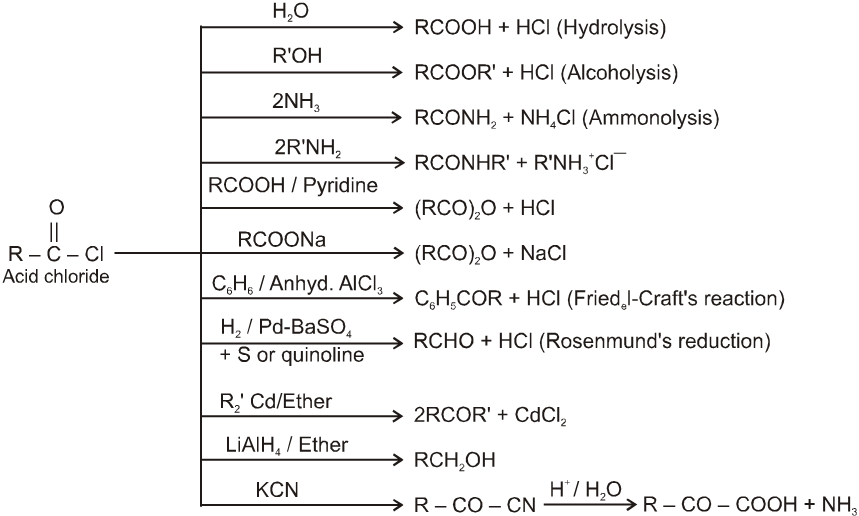
(B) Acid amides :
Methods of preparation of acids amides :
(1) By reaction of esters with ammonia and amines :
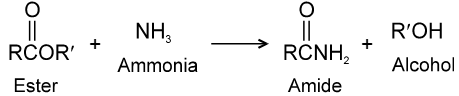
Ammonia is more nucleophilic than water, making it possible to carry out this reaction using aqueous ammonia.
(2) From acid halides :
RCOCl + 2NH3![]() RCONH2 + NH4Cl
RCONH2 + NH4Cl
(3) From anhydride :
(RCO)2O + 2NH3 ![]() RCONH2 + RCOONH4
RCONH2 + RCOONH4
(4) From ammonium salt of carboxylic acid :
RCOONH4 ![]() RCONH2 + H2O
RCONH2 + H2O
CH3COONH4 ![]() CH3CONH2
CH3CONH2
(5) From cyanides :
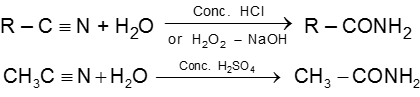
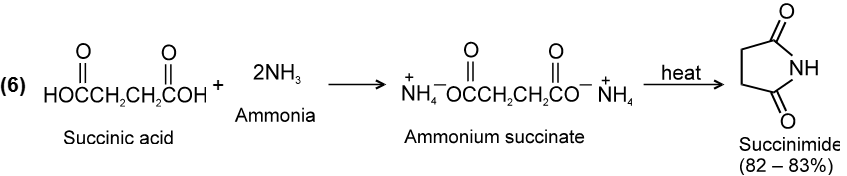
Chemical Reactions :
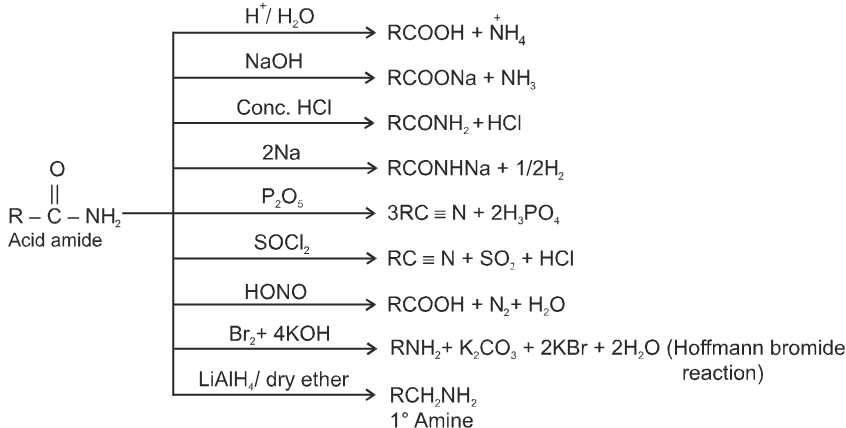
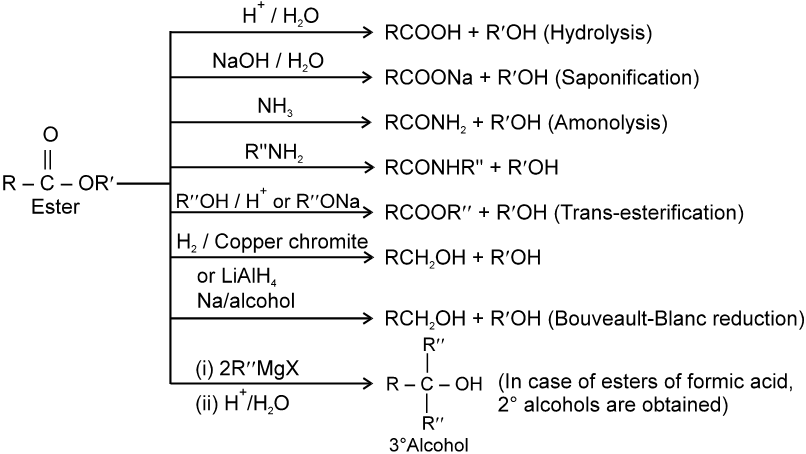
(C) Esters :
Methods of Preparation :
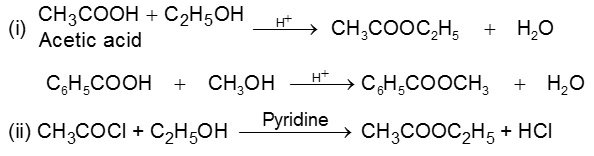
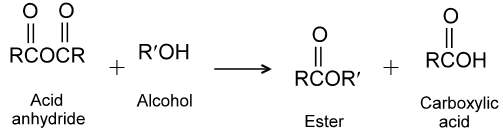
Alcohols react with acyl chlorides by nucleophilic acyl substitution to yield esters. These reactions are typically performed in the presence of a weak base such as pyridine.
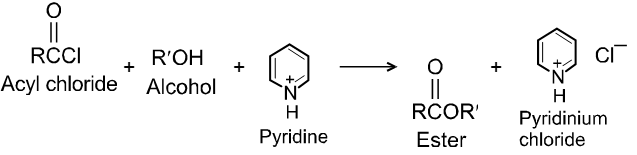
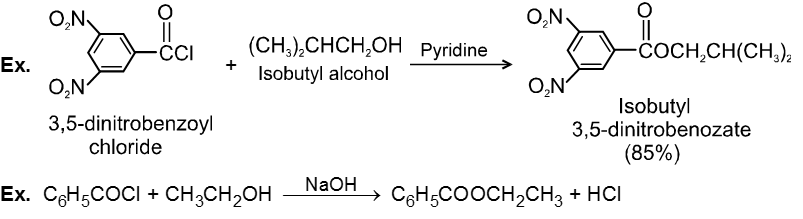
Chemical Reactions :
(D) Acid anhydrides :
Methods of Preparation of acid anhydrides :
(1) From carboxylic acids :
![]()
Ex. 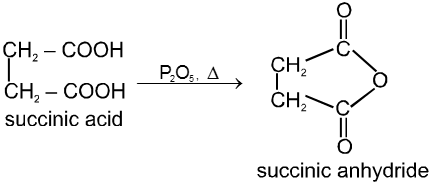
Ex.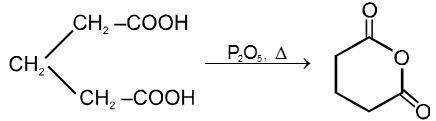
Ex. 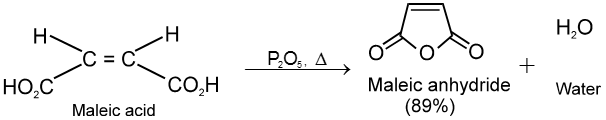
(2) From acid and acid halide :
Ex. ![]()
Ex. ![]()
Chemical Reactions
(1) Reaction with aromatic compounds (Friedel crafts acylation) :
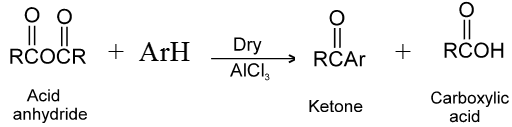
Ex.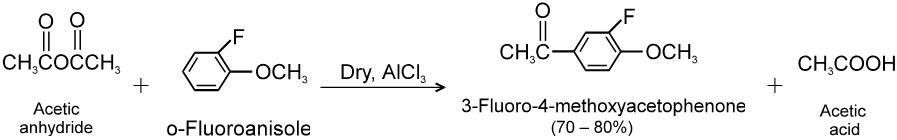
(2) Reaction with alcohols :
Ex.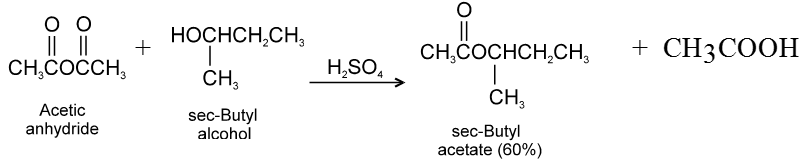
(3) Reaction with ammonia and amines :
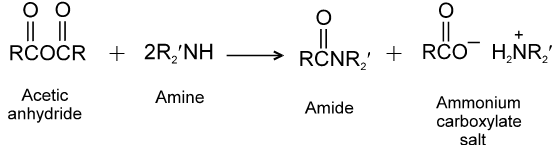
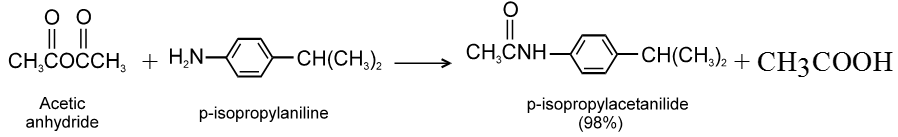
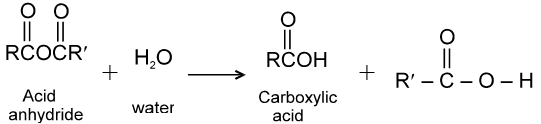
(4) Hydrolysis :
Acid anhydrides react with water to yield two carboxylic acid functions. Cyclic anhydrides yield dicarboxylic acids.
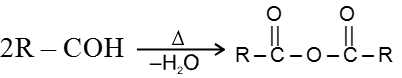
Heating Effects :
(a) Heating effect on monocarboxylic acid :
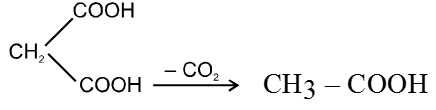
(b) Heating effect on dicarboxylic acid :
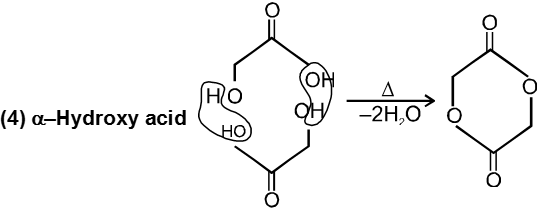
(c) Heating effects on Hydroxy acids :
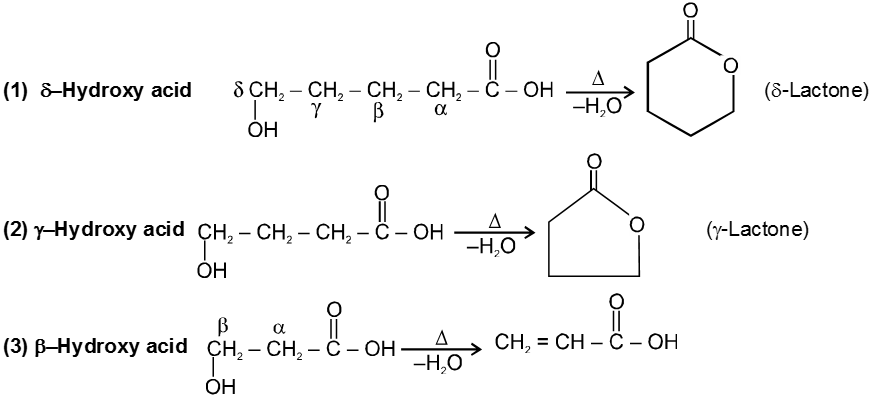
Since 4 or 8 membered rings are less stable the refore b-Hydroxy acids on heating produce a, b unsaturated carboxylic acid.
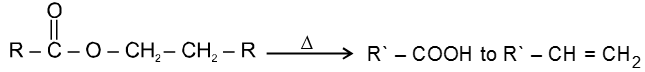
(d) Heating effects on esters :
Mech :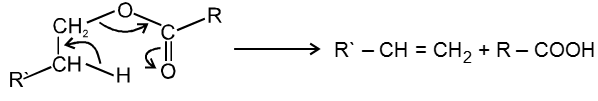
This reaction follows syn elimination & hoffman product is formed.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
