- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chemical Reactions of carboxylic acid
Acidic Nature : Acidity of carboxylic acids :
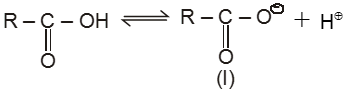
(i)![]()
(I) exists as two equivalent canonical structures I(A) and I(B). This ion is resonance stablised and resonance hybrid structure is I(C).
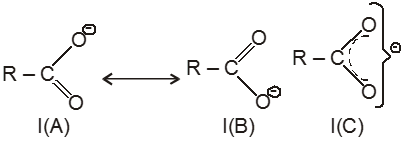
(ii) ion is more stable due to resonance, hence carboxylic acids are acidic in nature.
(iii) Electron withdrawing group (–I effect) stablises the anion and hence, increases acidic nature.
Ex. 
Ex. 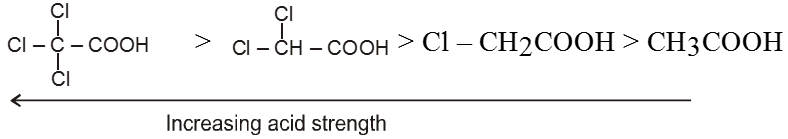
(iv) Electron releasing group (+ I effect) destablises the anion and hence decreases acidic nature.
Ex. HCOOH > CH3COOH > CH3 – CH2 – COOH
Ex. 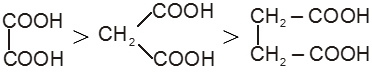
Direct attachement of groups such as phenyl or vinyl to the carboxylic acid increases its acidity.
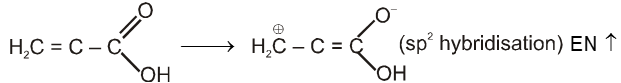
Ex. Relative acid strength is: RCOOH > HOH > ROH > HCCH > NH3 > RH
Note : Acidity of acids is compared by compairing stability of conjugate base.
(2) Reaction involving removal of proton from –OH group :
(i) Action with blue litmus : All carboxylic acids turn blue litmus into red.
(ii) Reaction with metals :
![]()
![]()
(iii) Reaction with alkalies :
CH3COOH + NaOH¾® CH3COONa + H2O
(iv) Reaction with carbonates and bicarbonates :
2CH3COOH + Na2CO3¾®2CH3COONa + CO2 + H2O
CH3COOH + NaHCO3¾®CH3COONa + CO2 + H2O
Reaction of carboxylic acid with aqueous sodium bicarbonate solution produces brisk effervescence. However most phenols do not produce effervescence. Therefore, the reaction may be used to distinguish between carboxylic acids and phenols.
(v) Reaction with grignard reagent :
![]()
Feasible reaction : A stronger acid displaces a weaker acid from the salt of the weaker acid.
Ex. CH3COOH (Stronger acid) + CH3ONa¾® CH3COONa + CH3–OH (Weaker Acid)
Ex. CH3COOH (stronger acid) + NaHCO3¾® CH3COONa + H2CO3 (Weaker acid) ¾® H2O + CO2![]()
(lab. test of carboxylic acid)
(3) Reaction involving replacement of –OH group :
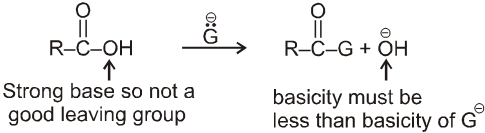
(i) Formation of acid chlorides :
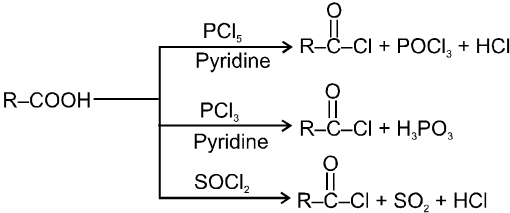
Ex.
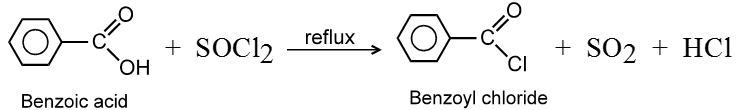
Ex.
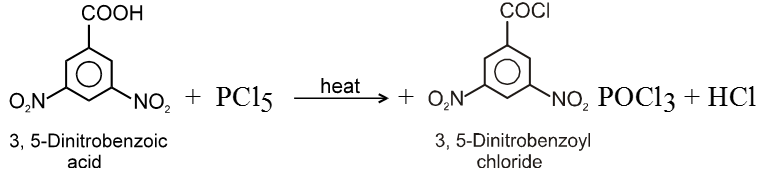
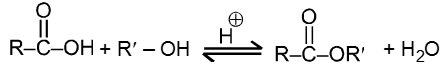
(ii) Fisher Esterification :
Carboxylic acid react with alcohol to form esters through a condensation reaction known as esterification.
General Reaction :
Specific Examples:
![]()
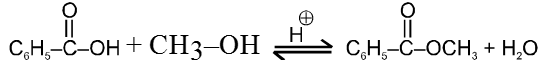
Mechanism : (Acid catalysed esterification)
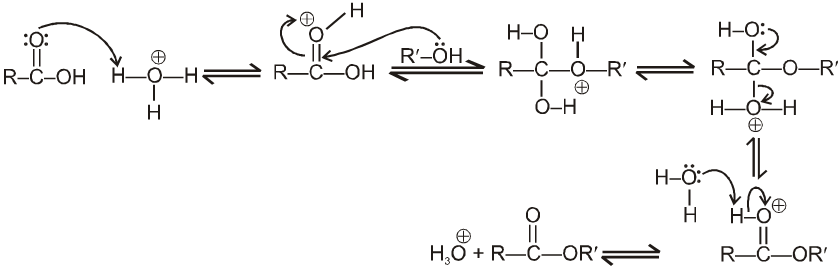
If we follow the forward reactions in this mechanism, we have the mechanism for the acid catalysed esterification of an acid. If however, we follow the reverse reactions, we have the mechanism for the acid catalysed hydrolysis of an ester.
Acid catalysed ester hydrolysis.
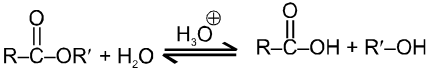
Which depends upon condition we choose. If we want to esterify an acid, use an excess amount of the alcohol and, if possible remove the water as it is formed.
If we want to hydrolyse an ester, we use a large amount of water i.e. dil HCl or dil H2SO4.
(iii) Formation of amides :
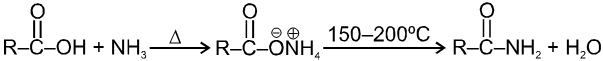
In fact amides can not be prepared from carboxylic acids and amines unless the ammonium salt is heated strongly to dehydrate it. This is not usually a good method of preparing amides.
(iv) Formation of acid anhydride :
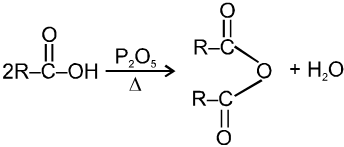
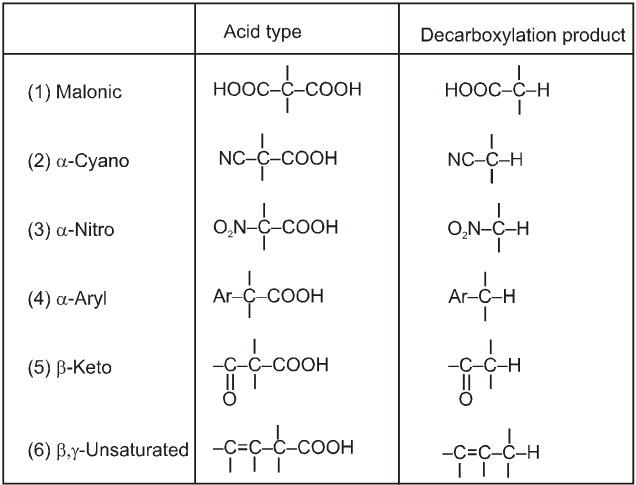
(4) Decarboxylation reactions :
(i)Soda-lime decarboxylation :
General reaction :
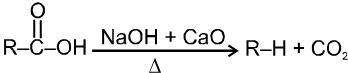
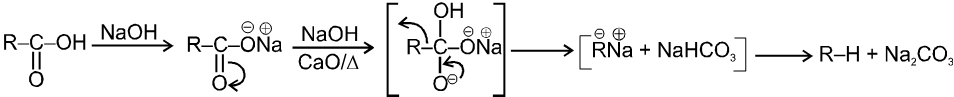
In this reaction carbanion intermediate is formed.
Rate of reaction depends upon the stability of carbanion intermediate.
Electron withdrawing group at R–COOH will increases the rate of decarboxylation.
Ex.
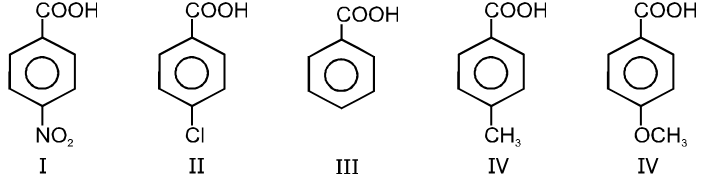
Rate of decarboxylation. I > II > III > IV > V
Ex.
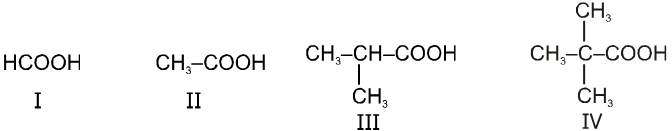
Rate of decarboxylation. I > II > III > IV
(ii) Decarboxylation of b-keto carboxylic acids:
b-keto acids decarboxylate readily when they are heated to 100–150ºC.
![]()
There are two reasons for ease of decarboxylation.
When the acid itself decarboxylates, it can do so through a six membered cyclic transition state :
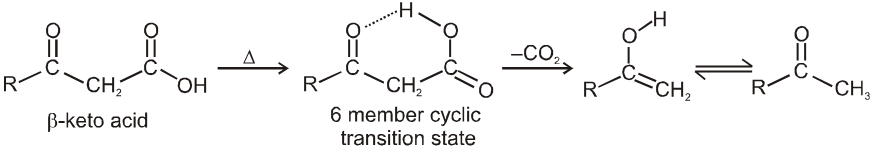
This reaction produces an enol directly and avoids an anionic intermediate. The enol then tautomerises to a methyl ketone.
When the carboxylate anion decarboxylates, it forms a resonance stabilized enolate anion.
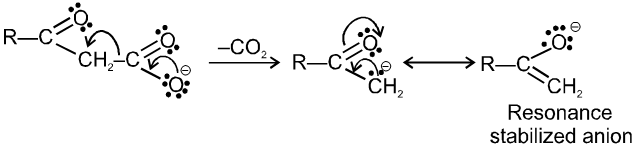
Alphatic acids that do undergo successful decarboxylation have certain functional groups or double or triple bonds in the a or b positions.
(iii) Kolbe’s electrolysis
![]()
If n is the number of carbon atoms in the salt of carboxylic acid, the alkane formed has 2(n–1) carbon atoms.
Ex.
![]()
(iv) Hunsdiecker Reaction (Bromo-decarboxylation) :
R–COOAg + Br2 ¾®R–Br + CO2 + AgBr
Heavy metal salt of acid is heated with Br2 it results in decarboxylations.
Metal can be silver ion, mercuric ion, or lead (IV) ion.
Mechanism :
Step 1 : ![]()
Step 2 : ![]()
Step3 : ![]()
Step 4 : ![]()
Although bromine is the most often used halogen, chlorine and iodine have also been used.
When iodine is the reagent, the ratio between the reactant is very important and determines the product.
A 1 : 1 ratio of salt to iodine gives alkyl halide, as above. A 2 : 1 ratio, however gives the ester RCOOR. This is called Simonini reaction and sometimes used to prepare carboxylic ester. The yield of alkyl halide follows the order Primary > Secondary > Tertiary
(5) HVZ Reaction (Halogenation of aliphatic acids and Substituted acids) :
In the presence of a small amount of phosphorus, aliphatic carboxylic acids react smoothly with chlorine or bromine to yield a compound in which -hydrogen has been replaced by halogen.
![]()
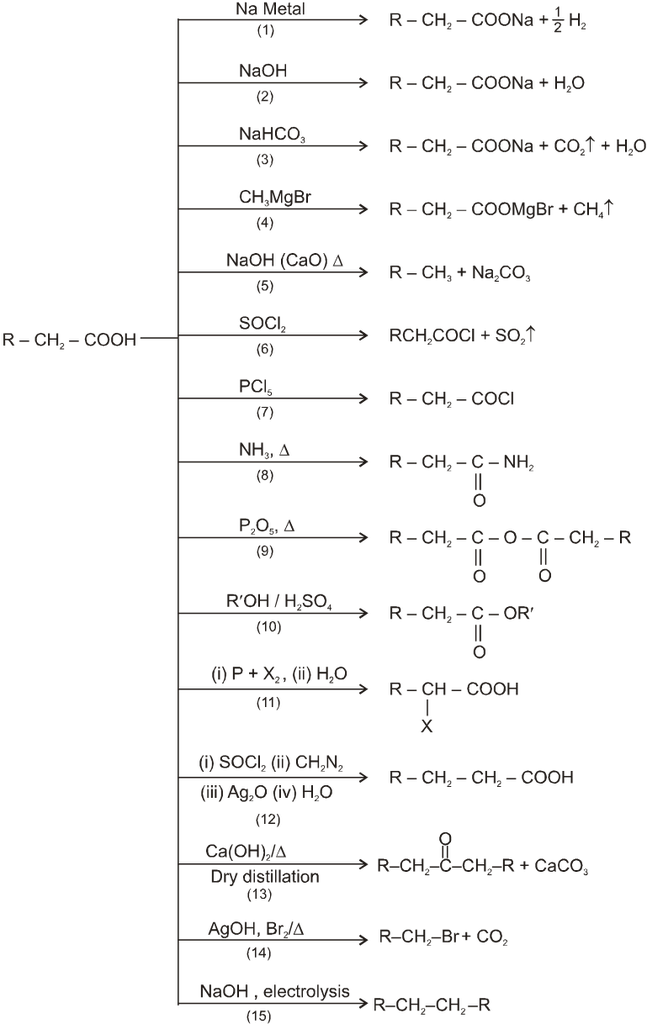
Summary of reactions of carboxylic acids :

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
