- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Group 16 Elements : The Oxygen family
Oxygen, sulphur, selenium, tellurium and polonium constitute group 16 of the periodic table. This is sometimes known as group of chalcogens the ore forming elements because a large number of metals ores are oxides or sulphides.
Electronic Configuration : The elements of group 16 have six electrons in the outermost shell and have ns2 np4 general valence shell electronic configuration.
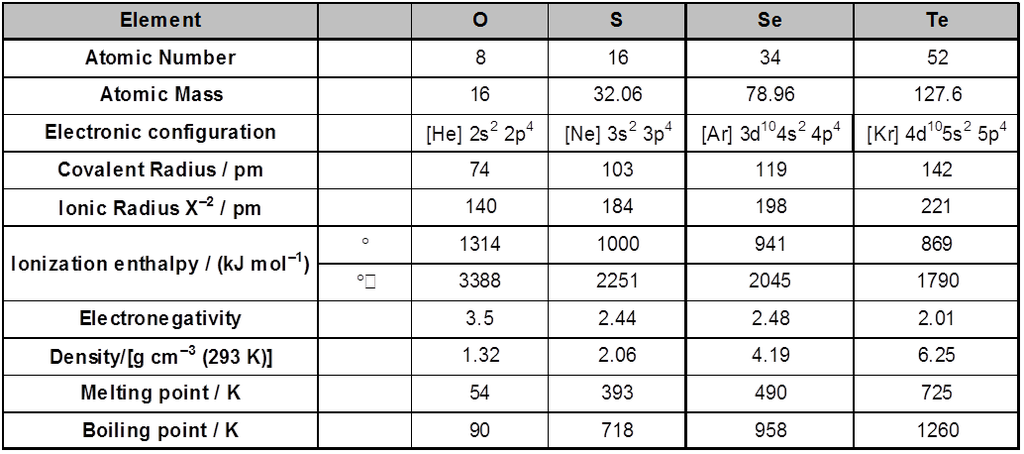
Atomic and Ionic Radii : Due to increase in the number of shells , atomic and ionic radii increase from top to bottom in the group. The size of oxygen atoms is however, exceptionally small .
Ionisation Enthalpy : Ionisation enthalpy decreases down the group. It is due to increase in size. However, the element of this group have lower ionisation enthalpy values compared to those of group 15 in the corresponding periods. This is due to the fact that group 15 elements have extra stable half-filled p orbitals electronic configurations.
Electron Gain Enthalpy : Because of the compact nature of oxygen atom, it has less negative electron gain enthalpy than sulphur. However from sulphur onwards the value again becomes less negative upto polonium.
Electronegativity : Next to fluorine, oxygen has the highest electronegativity value amongst the elements. Within the group, electronegativity decrease with an increase in atomic number. This indicates that the metallic character increases from oxygen to polonium.
Physical Properties : Oxygen and sulphur are non-metal, selenium and tellurium metalloids, whereas polonium is a metal. Polonium is radioactive and is short lived (Half-life 13.8 days). The melting and boiling points increase with an increase in atomic number down the group. The larger difference between the melting and boiling points of oxygen and sulphur may be explained on the basis of their atomicity; oxygen exist as diatomic molecules (O2) whereas sulphur exists as polyatomic molecule (S8).
Catenation : Tendency for catenation decreases down the group. This property is prominently displayed by sulphur (S8). The S—S bond is important in biological system and is found in some proteins and enzymes such as cysteine.
Selenium has unique property of photo conductivity and is used in photocopying machines and also a decolouriser of glass.
Atomic & Physical Properties
Chemical Properties :
Oxidation states and trends in chemical reactivity :
The elements of group 16 exhibit a number of oxidation states. The stability of -2 oxidation state decreases down the group. Polonium hardly shows -2 oxidation states. Since electronegativity of oxygen is very high, it shows only negative oxidation states as -2 except in the case of OF2 where its oxidation states is + 2. Other elements of the group exhibit + 2 + 4 + 6 oxidation states but + 4 and + 6 are more common. Sulphur, selenium and tellurium usually show + 4 oxidation in their compounds with oxygen and +6 oxidations state with fluorine. The stability of +6 oxidation state decreases down the group and stability of + 4 oxidation state increases (inert pair effect). Bonding in + 4 and + 6 oxidation states are primarily covalent.
HNO3 oxidises sulphur to H2SO4 (S + VI) but only oxidises selenium to H2SeO3 (Se + IV) as the atoms are smaller and there is poor shielding of 3d electrons as a result the electrons are held more tightly with nucleus.
Anomalous behaviour of oxygen :
The anomalous behaviour of oxygen, like other member of p-block present in second period is due to its small size and high electronegativity. One typical example of effects of small size and high electronegativity is the presence of strong hydrogen bonding in H2O which is not found in H2S.
The absence of d orbitals in oxygen restricts its covalency to four and in practice, rarely increases beyond two. On the other hand, in case of other elements of the group, the valence shell can be expanded and covalence exceeds four.
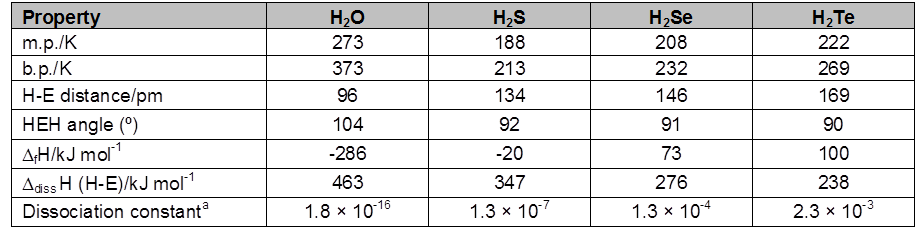
(i) Reactivity with hydrogen : All the elements of group 16 form hydrides of the type H2E (E = S, Se, Te, Po). Some properties of hydrides are given in Table. Their acidic character increases from H2O to H2Te. The increase in acidic character can be understood in terms of decrease in bond (H-E) dissociation enthalpy down the group. Owing to the decrease in bond (H-E) dissociation enthalpy down the group , the thermal stability of hydrides also decreases from H2O to H2Po. All the hydrides except water possess reducing property and this property increases from H2S to H2Te.
Table : Properties of Hydrides of Group 16 Elements
(ii) Reactivity with oxygen : All these elements form oxides of the EO2 and EO3 types where E = S, Se, Te or Po. Ozone (O3) and sulphur dioxide (SO2) are gases while selenium dioxide (SeO2) is solid. Reducing property of dioxide decreases from SO2 to TeO2 ; SO2 is reducing while TeO2 is an oxidising agent. Besides EO2 type sulphur, selenium and tellurium also form EO3 type oxides (SO3, SeO3, TeO3). Both types of oxides are acidic in nature.
(iii) Reactivity toward the halogens : Elements of group 16 form a larger number of halides of the type EX6, EX4 and EX2 where E is an element of the group and X is an halogen. The stabilities of the halides decrease in the order F > Cl > Br > l. Amongst hexahalides, hexafluorides are the only stable halides. All hexafluorides are gaseous in nature. They have octahedral structure. Sulphur hexafluoride SF6 is exceptionally stable for steric reasons.
Amongst tetrafluorides, SF4 is a gas , SeF4 liquid and TeF4 a solid.
All elements except selenium form dichlorides and dibromides.The well known monohalides are dimeric in nature, Examples are S2F2, S2Cl2, S2Br2, Se2Cl2 and Se2Br2. These dimeric halides undergo disproportionation as given below :
2Se2Cl2 ® SeCl4 + 3Se.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
