- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 7
p-block (nitrogen and oxygen family)
Introduction :
Group 13 to 18 of the periodic table of elements constitute the p–block. The p–block contains metals, metalloids as well as non–metals.
The p–block elements have general valence shell electronic configuration ns2 np1–6.
The first member of each group from 13–17 of the p–block elements differ in many respects from the other members of their respective groups because of small size, high electronegativity and absence of d–orbitals.
The first member of a group also has greater ability to form pp–pp multiple bonds to itself (e.g. C=C, CºC, NºN) and to element of second row (e.g C=O, C=N, CºN, N=O) compared to the other members of the same group.
The highest oxidation of p–block element is equal to the group number minus 10. Moving down the group, the oxidation state two less than the highest group oxidation state becomes more stable in groups 13 to 16 due to inert pair effect (reluctance of s-subshell electrons to participate in chemical bonding)
Group 15 Elements : The Nitrogen family
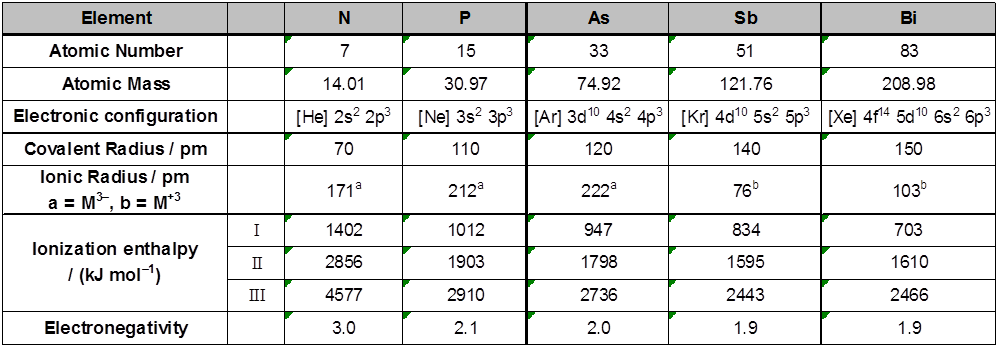
Group 15 includes nitrogen phosphorus, arsenic, antimony and bismuth. As we go down the group, there is a shift from non-metallic to metallic through metalloidic character. Nitrogen and phosphorus are non-metal, arsenic and antimony metalloid and bismuth is a typical metal.
Electronic Configuration : The valence shell electronic configuration of these element is ns2 np3. The s orbital in these element is completely filled and p orbitals are half- filled, making their electronic configuration extra stable.
Atomic and Ionic Radii : Covalent and ionic (in a particular state) radii increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and / or f orbitals in heavier members.
Ionisation Enthalpy : Ionisation enthalpy decreases down the group due to gradual increase in atomic size. Because of the extra stable half- filled p-orbital electronic configuration and smaller size, the ionisation enthalpy of the group 15 element is much greater than of group 14 elements in the corresponding periods. The order of successive ionisation enthalpies, as expected is DiH1 < DiH2 < DiH3
Electronegativity : The electronegativity value, in general, decreases down the group with increasing atomic size. However, amongst the heavier elements, the difference is not that much pronounced.
Physical Properties : All the elements of this group are polyatomic. Dinitrogen is a diatomic gas while all others are solids. Metallic character increases down the group. Nitrogen and phosphorus are non–metals, arsenic and antimony metalloids and bismuth is a metal. This is due to decrease in ionisation enthalpy and increase in atomic size. The boiling points, in general, increase from top to bottom in the group but the melting point increases upto arsenic and then decreases upto bismuth. Except nitrogen, all the elements show allotropy.
Atomic & physical properties
Chemical Properties :
Oxidation States and trends in a chemical reactivity :
The common oxidation states of these elements are –3, +3 and +5. The tendency to exhibit –3 oxidation state decreases down the group , bismuth hardly forms any compound in –3 oxidation state. The stability of +5 oxidation state decreases down the group. The only well characterised Bi (V) compound is BiF5 .The stability of +5 oxidation state decreases and that of +3 state increases (due to inert pair effect) down the group.
Bi3+ > Sb3+ > As3+ ; Bi5+ < Sb5+ < As5+
Nitrogen exhibits +1, +2, +4 oxidation states also when it reacts with oxygen. Phosphorus also shows +1 and +4 oxidation states in some oxoacids.
In the case of nitrogen, all oxidation states from +1 to +4 tend to disproportionate in acid solution.
For example,
3 HNO2 —® HNO3 + H2O + 2 NO
Similarly, in case of phosphorus nearly all intermediate oxidation states disproportionate into +5 and –3 both in alkali and acid. However +3 oxidation state in case of arsenic , antimony and bismuth become increasingly stable with respect to disproportionation.
Nitrogen is restricted to a maximum covalency of 4 since only four (one s and three p) orbitals are available for bonding. The heavier elements have vacant d orbitals in the outermost shell which can be used for bonding (covalency) and hence , expand their covalence as in PF6– .
Anomalous properties of nitrogen :
Nitrogen differs from the rest of the members of this group due to its smaller size, high electronegativity, high ionisation enthalpy and non–availability of d orbitals. Nitrogen has unique ability to form pp–pp multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form pp–pp bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a diatomic molecule with a triple bond (one s and two p) between the two atoms. Consequently, its bond enthalpy (941.1 kJ mol–1) is very high. On the contrary, phosphorus, arsenic and antimony form metallic bonds in elemental state. However, the single N–N bond is weaker than the single P–P bond because of high interelectronic repulsion of the non–bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen. Another factor which affects the chemistry of nitrogen is the absence of d orbitals in its valence shell. Besides restricting its covalency to four, nitrogen cannot form dp–pp bonds as the heavier elements can e.g., R3P=O or R3P=CH2 (R = alkyl group). Phosphorus and arsenic can form dp–pp bond also with transition metals when their compounds like P(C2H5)3 and As(C6H5)3 act as ligands.
(i) Reactivity towards hydrogen : All the elements of Group 15 form hydrides of the type EH3 where E=N, P, As, Sb or Bi. Some of the properties of these hydrides are shown in Table. The hydrides show regular gradation in their properties. The stability of hydrides decreases from NH3 to BiH3 which can be observed from their bond dissociation enthalpy. Consequently, the reducing character of the hydrides increases. Ammonia is only a mild reducing agent while BiH3 is the strongest reducing agent amongst all the hydrides. Basicity also decreases in the order NH3 > PH3 > AsH3 > SbH3 ³ BiH3 .
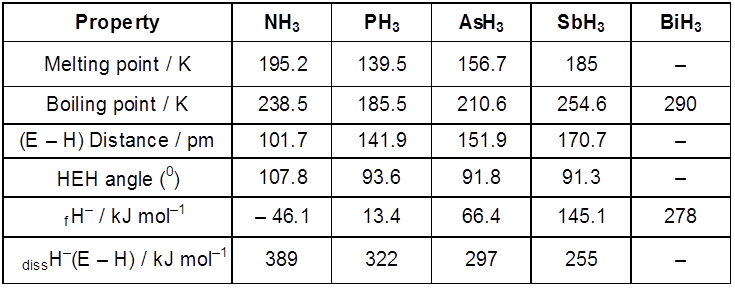
Properties of Hydrides of Group 15 Elements
(ii) Reactivity towards oxygen : All these elements form two types of oxides : E2O3 and E2O5 . The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state. Their acidic character decreases down the group. The oxides of the type E2O3 of nitrogen and phosphorus are purely acidic , that of arsenic and antimony amphoteric and those of bismuth is predominantly basic.
(iii) Reactivity towards halogens : These elements react to form two series of halides : EX3 and EX5 . Nitrogen does not form pentahalide due to non – availability of the d-orbitals in its valence shell. Pentahalides are more covalent than trihalides. All the trihalides of these elements except those of nitrogen are stable. In case of nitrogen, only NF3 is known to be stable. Trihalides except BiF3 are predominantly covalent in nature. Halides are hydrolysed in water forming oxyacids or oxychlorides.
PCl3 + H2O —® H3PO3 + HCl
SbCl3 + H2O —® SbOCl¯ (orange) + 2HCl
BiCl3 + H2O —® BiOCl¯ (white) + 2HCl
(iv) Reactivity towards metals : These elements react with metals to form their binary compounds exhibiting –3 oxidation state , such as , Ca3N2 (calcium nitride) Ca3P2 (calcium phosphide) , Na3As2 (sodium arsenide), Zn3Sb2 (zinc antimonide) and Mg3Bi2 (magnesium bismuthide).

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
