- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 14: biomolecules & polymers
Carbohydrates (Saccharides) : (Latin word : saccharum = sugar)
Concept
Generally, carbohydrates are substances with the general formula Cx(H2O)y , and were therefore called carboydrates (hydrates of carbon) because they contained hydrogen and oxygen in the same proportion as in water. How ever, a number of compounds have been discovered which are carbohydrates by chemical behaviour but do not conform to the formula Cx(H2O)y e.g ,2-deoxyribose, C5H10O4.
Hence, carbohydrates are biopolymers of polyhydroxy aldehyde or polyhydroxy ketones. “There monomeric polyhydroxy aldehydes or ketones can also exist in hemiocetal and acetal forms in cyclic structures.”
# Note:
Almost all of these compounds are chiral & optically active. “An exception of this is 1,3-dihydroxypropanone, CH2OHCOCH2OH”.
2 Classification of carbohydrates :
(A) Classification on the basis of number of hydrolysed products

(B) On the Basic of functional group
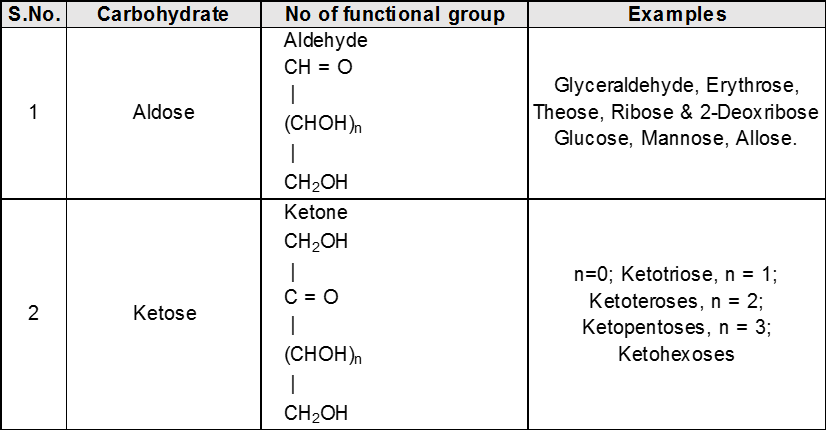
(C) On basis of carbon atoms.

Configuration of the D-Aldoses
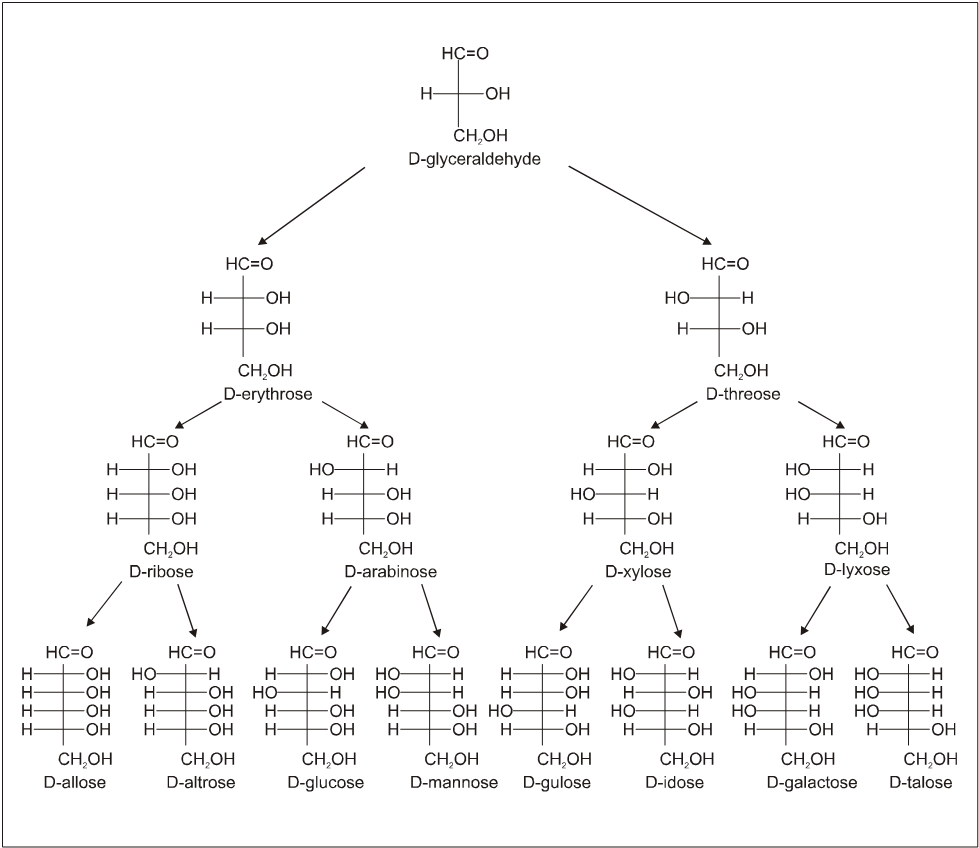
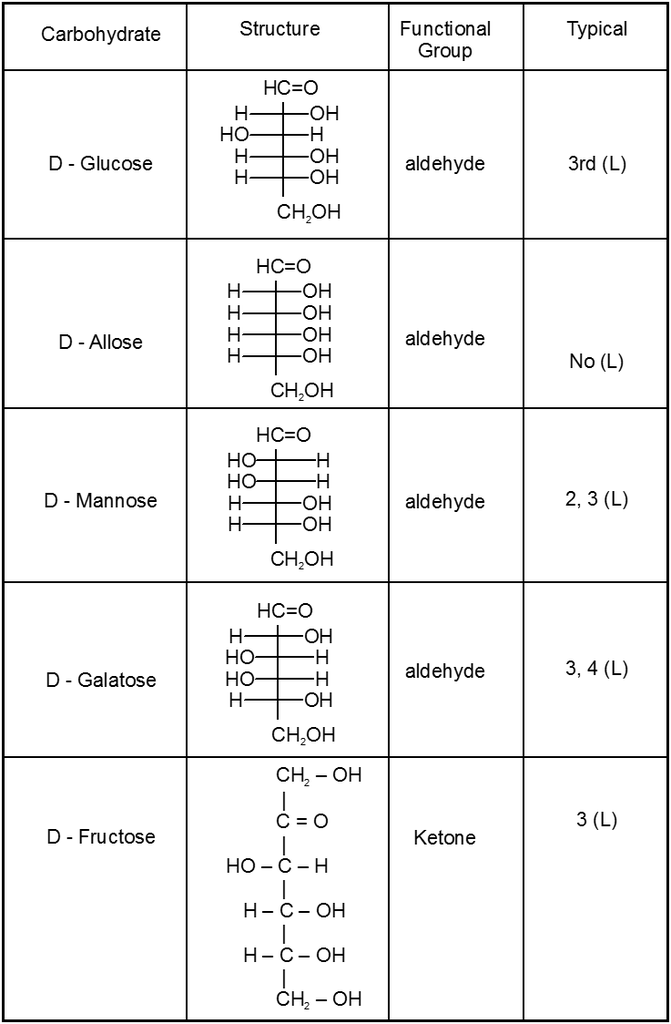
3. Structure :
(i) All form of Aldose or ketose may exist in open chain form as well as in cyclic pyramose/furanose form.
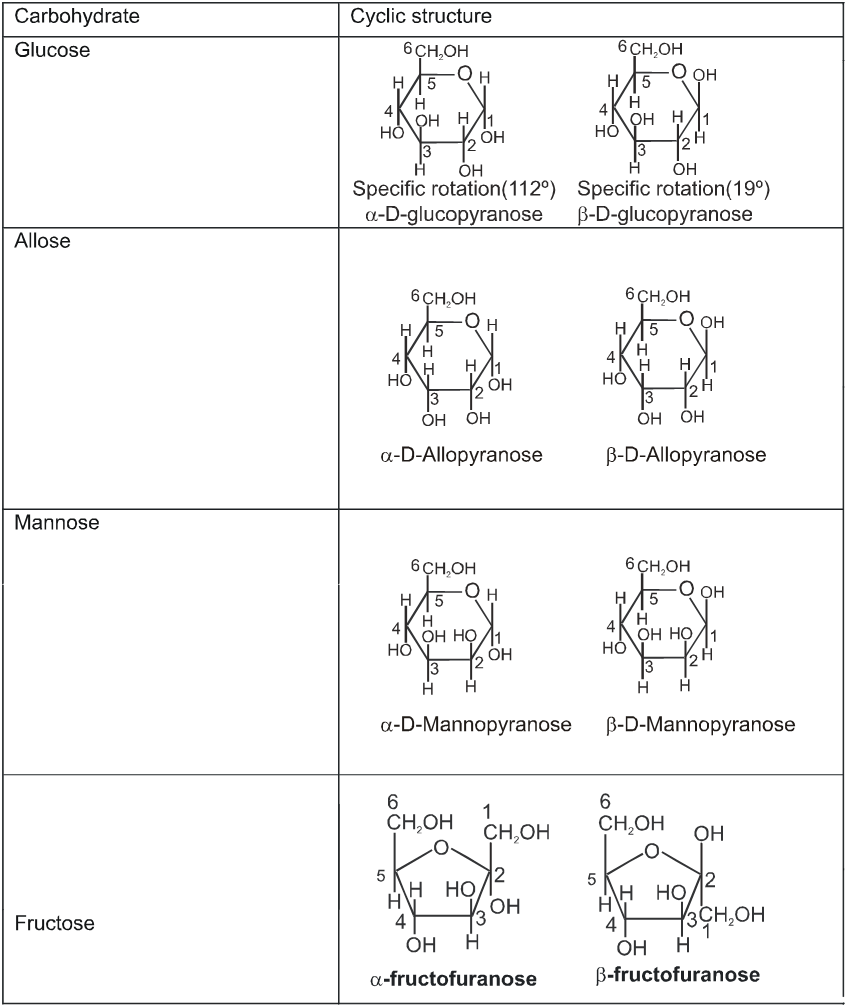
Cyclie structure of monosaccharide
Disaccharide
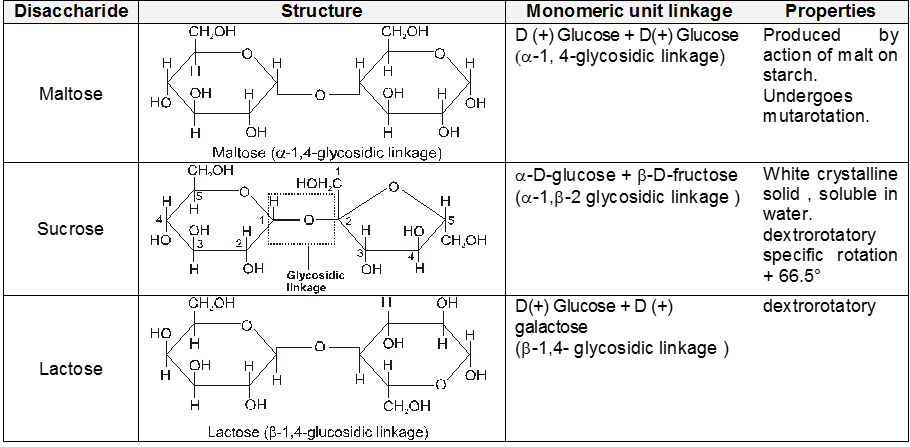
Polysaccharide
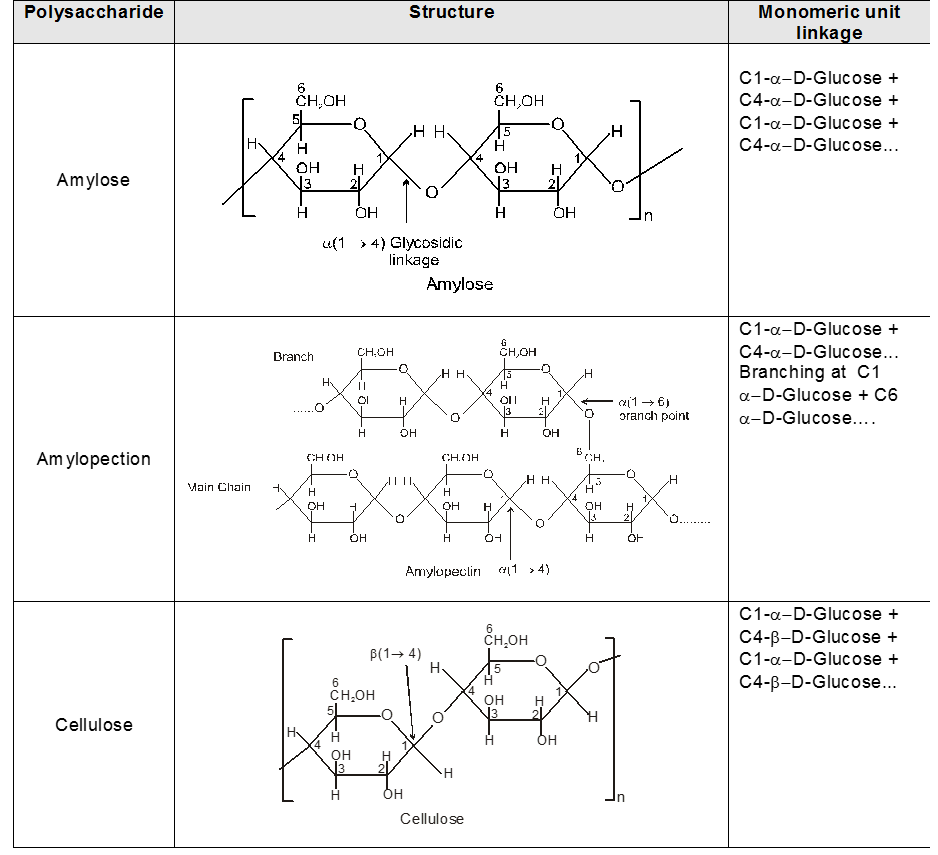
INTER CONVERSION OF a and b
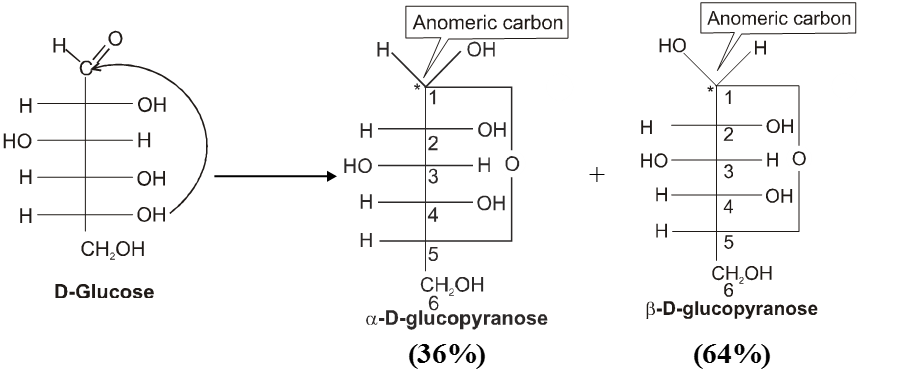
Haworth projection
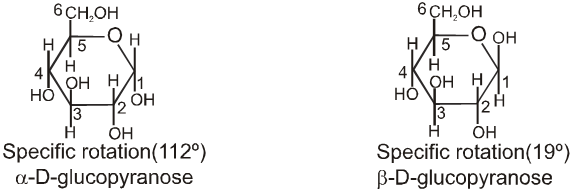
Chair conformation structures :
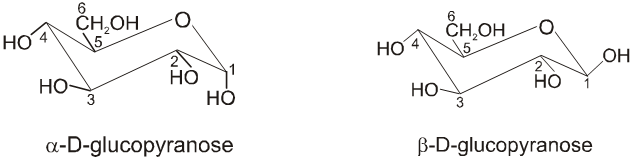
Anomers :
Anomers are diastereomers that differ in the configuration at the acetal or hemiacetal C atom of a sugar in its cyclic form or Anomers are epimers whose conformations differ only about C-1.
For example, a D(+) and b -D(+) glucose are anomers. a-D(–) and b-D(–) fructose are anomers.
Epimers :
Diastereomers with more than one stereocentre that differ in the configuration about only one stereocentre are called epimers.
i. D-Glyceraldehyde and L-glyceraldehyde (this pair is an enantiomer as well as an epimer).
ii. D-Erythrose and L-threose are epimers.
iii. D-glucose and D-galactose are C-4 epimers and
iv. D-idose and D-talose are C-3 epimers.
v. D-glucose and D-mannose are C-2 epimers.
vi. Epimerisation of glucose at C-2 gives mannose.
vii. Epimerisation of glucose at C-3 gives allose.
viii. Epimerisation of glucose at C-4 gives galactose.
Action of alkalies :
(a) With conc. NaOH glucose first turns yellow resinify. Fructose is not effected by conc. NaOH
(b) With dil. NaOH/KOH both Glucose & Fructose undergo a rearrangement to give equilibrium mixture of three sugars.
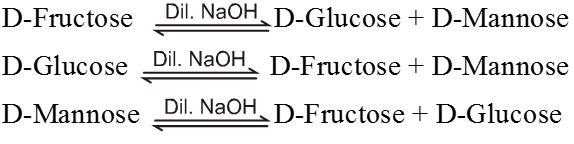
This rearrangement is known as Lobry De-bryn. van Ekenstein rearrangement.
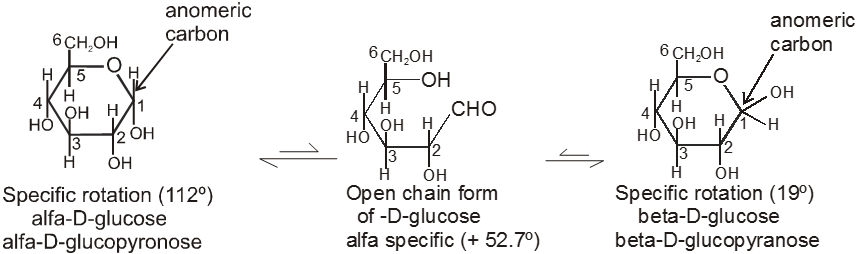
4. Properties of Anomers : Mutarotation
When one of the pure glucose anomers dissolve in water, an intersting change in the specific rotation is observed. When the a-anomer dissolves, its specific rotation gradually decreases from an initial value of +112º to +52.7º. When the pure b- anomer dissolves, its specific rotation gradually increases from +19º to the same value of +52.7º. This change (mutation) in the specific rotation is called mutarotation. What is happening is that each solution, initially containing only one anomeric form, undergoes equilibrium to the same mixture of a-and b-forms. The open chain forms is in intermediate in the process.
For mutarotation atleast one hemiacetal group must be present in the sugar therefore all reducing sugars will mutarotate.
(C) Classification on the basis of Reducing and non Reducing properties :
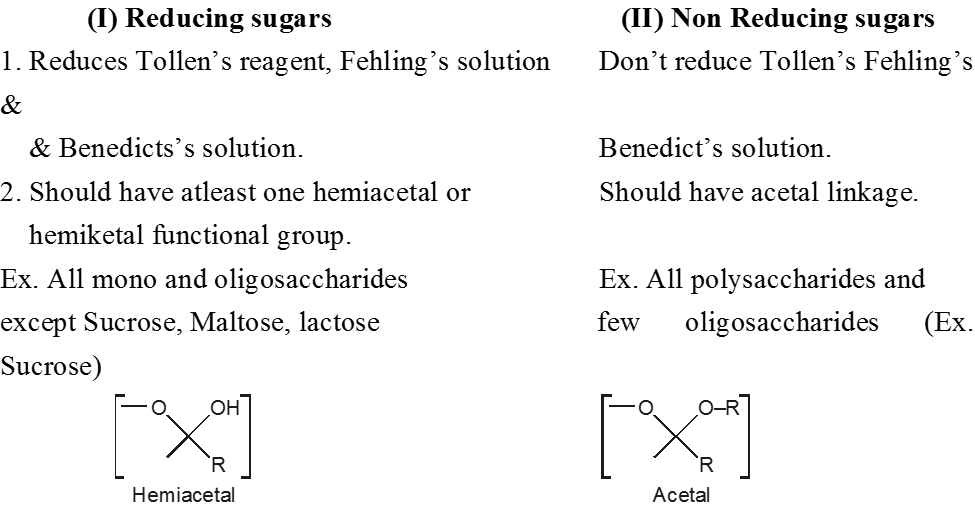
Monosaccharides :
(A) Glucose (C6H12O6) :
Glucose is the most common monosaccharide. It is known as Dextrose because it occurs in nature principally as the optically active dextrorotatory isomers. It is act as a reducing agent (reduces both Fehling’s solution and ammonical silver nitrate solution). When heated with sodium hydroxide, an aqueous solution of glucose turns brown. It is known as dextrose and found as grapes, honey, cane sugar, starch and cellulose.
Preparation :
(1) By acid hydrolysis of cane sugar (a disaccharide) :
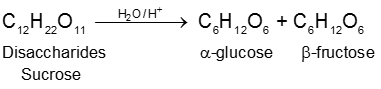
a-Glucose being much less soluble in alcohol than fructose separate out by crystallization on cooling.
(2) By enzymatic action over starch :
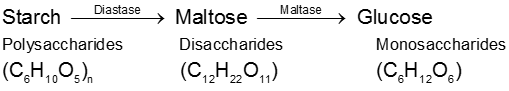
Physical properties :
(i) It is white crystalline solids having melting point 146ºC. It is readily soluble in water.
(ii) Glucose is sweet in taste and also optically active (dextro rotatory).
(iii) Glucose shows mutarotation.
Chemical properties :
(1) Reactions due to OH group :
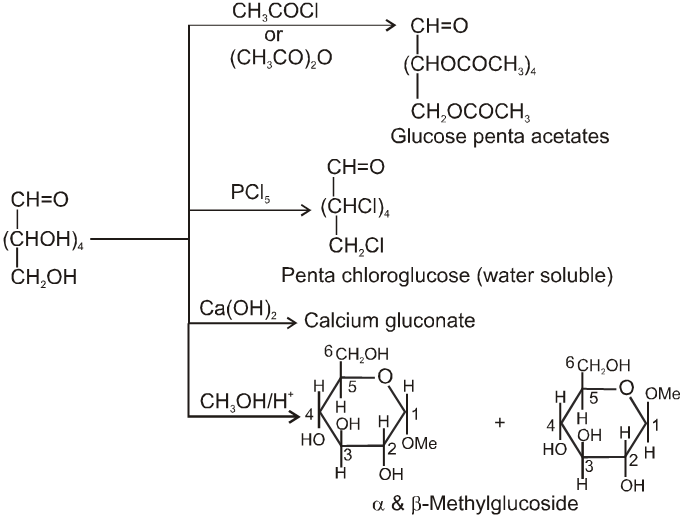
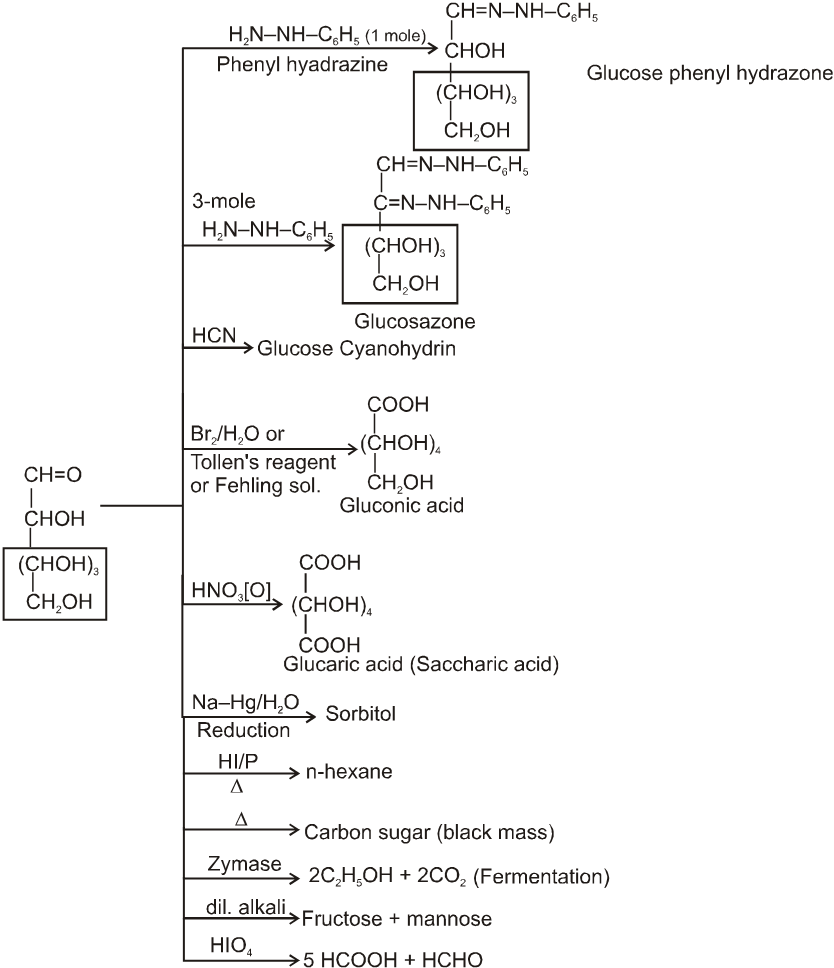
(2) Reactions due to –CHO group :
Glycosides-formation :
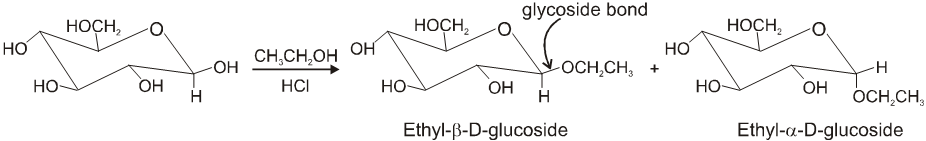
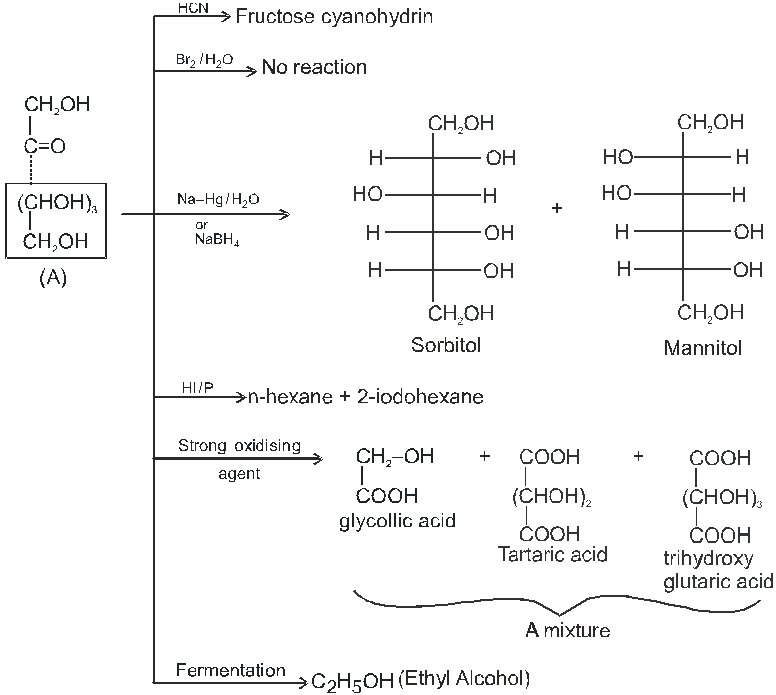
(B) Fructose :
- Also said to be fruit sugar
- It occur both in combined as well as free state.
- Fructose is named as fruit sugar because it is present in honey and most sweet fruit in free state.
- It is sweetest monosaccharide and present in cane sugar and insulin in combined state.
- It is also known as a-Laevulose i.e. natural occurring compound is laevorotatory
Preparation :
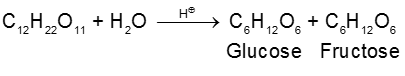
By acid hydrolysis of cane sugar :
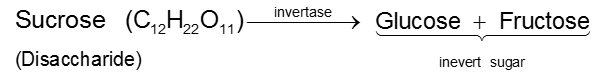
By enzymatic action of sucrose
Note: Glucose and fructose obtained by acid hydrolysis of sucrose can be separated by treating with Ca(OH)2 which forms calcium glucosate & calcium fructosate. Calcium fructosate being water insoluble. It is seperated out easily
Physical properties :
(1) It is colourless crystalline solid.
(2) It is soluble in water but insoluble in ether ketone and benzene.
(3) It is pentahydroxy ketone and shows mutarotation like glucose.
(4) It is reducing sugar.
Chemical Properties :
Due to OH group:
(1) It froms fructose penta acetate with acetyl chloride :
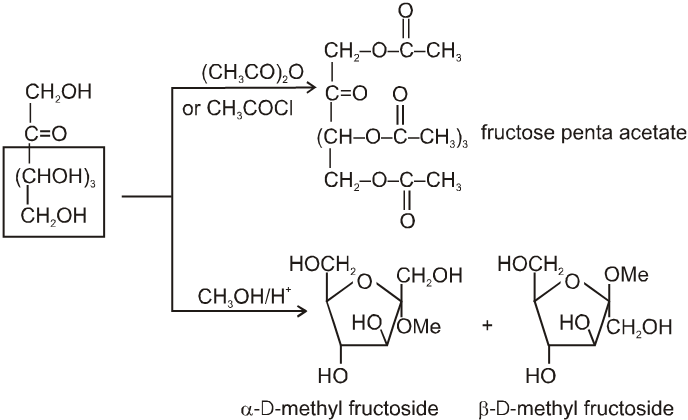
(2) Reaction due to keto group :
It also form osazone with excess of phenyl hydrazine thus we can say that osazone formation is characteristic of a-hydroxy carbonyl compounds.
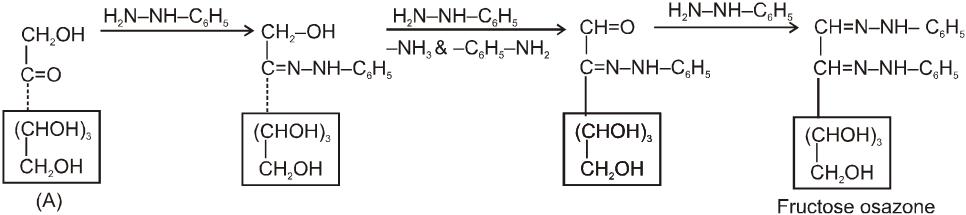
Note : 1. Unlike other ketones, fructose can reduced Fehling sol. and Tollen’s reagents it is probably due to formation of an equilibrium between glucose, mannose and fructose.
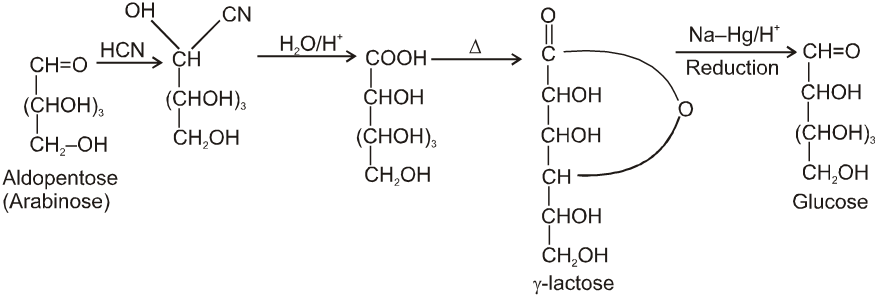
Chain lengthing :
Chain shortening :
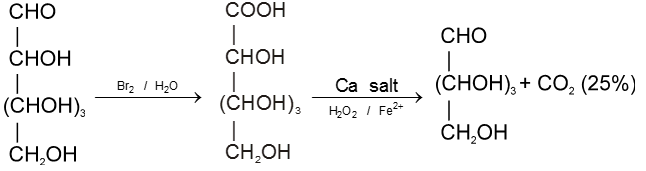
Disaccharides :-
(A) Sucrose : (Sucrose, Invert-sugar C12H22O11)
(a) Sucrose (Cane sugar) ![]() a–glucose + b-fructose
a–glucose + b-fructose
Formation of sucrose (C12H22O11)
a–D-glucose + b-D-fructose
C1 C2
Pyranose form Furanose form
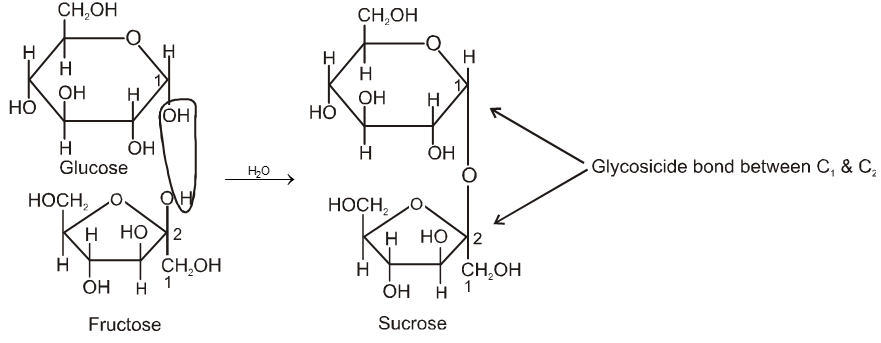
Two monosaccharides are joined together by an oxide linkage formed by loss of water molecule. Such linkage through oxygen atom is called glycosidic linkage.
In sucrose linkage in between C1 of a-glucose and C2 of b-fructose. Since the reducing group of glucose & fructose are involved in glycosidic bond formation, sucrose is non reducing sugar.
(i) On hydrolysis with dilute acids sucrose yields an equimolecular mixture of D(+)-glucose and
D(–)-fructose :
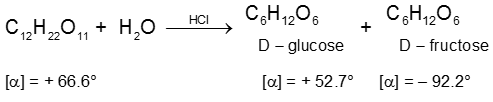
Since D(–)-fructose has a greater specific rotation than D(+)-glucose, the resulting mixture is laevorotatory. Because of this, hydrolysis of cane-sugar is known as the inversion of cane-sugar or Inversion of sucrose (this is not to be confused with the Walden inversion), and the mixture of sugars are known as invert sugar Ex. D - Glucose & D-Fructose. The inversion (i.e., hydrolysis) of cane-sugar may also be effected by the enzyme invertase which is found in yeast.
(ii) Sucrose is not a reducing sugar, e.g., it will not reduce Fehling’s solution or Tollen’s reagnet. It does not form an oxime or an osazone, and does not undergo mutarotation. This indicates that hemiacetal group is not present in the rings.
Polysaccharides :-
(A) Starch (C6H10O5)n :
(i) Starch is the main contributor of carbohydrates in our diet. It exists exclusively in plants, stored in the seeds, roots, and fibres as food reserve. Example rice, potato.
(ii) Both amylose and amylopectin are composed of D-glucose units.
(iii) The amylose molecule is made up of D-glucose unit joined by a-glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose unit. The number of D-glucose units in amylose range from 60-300.
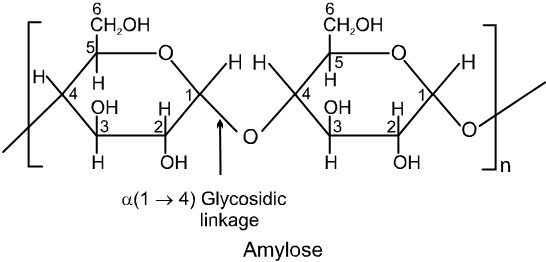
(iv) Amylopectin has a branched-chain structure. It is composed of chains of 25 to 30 D-glucose units joined by a-glycosidic linkages between C-1 to one glucose unit and C-4 of the next glucose unit. These chains are in turn connected to each other by 1, 6-linkages.
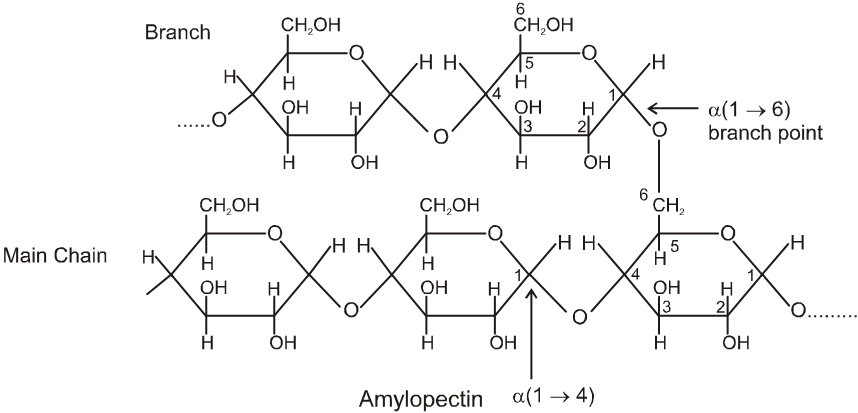
The solution of a-amylose gives a blue colour with iodine.
The solution of Amylopectin gives a violet colour with iodine :
(B) Cellulose, (C6H10O5)n :
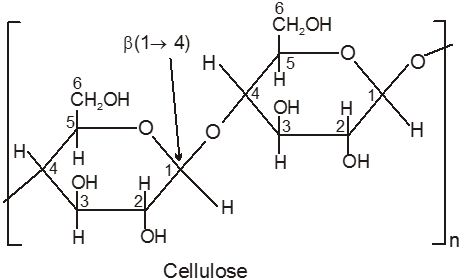
(i) Cellulose is the main structural material of trees and other plants. Wood is 50% cellulose, while cotton and wool are almost pure cellulose. Other sources of cellulose are straw, corncobs, bagasse, and similar agricultural wastes.
(ii) Artificial silk, rayon, is used collectively to cover all synthetic or manufactured fibres from cellulose.
(iii) The nitrates are prepared by the reaction of cellulose with a mixture of nitric and sulphuric acids, and the degree of ‘nitration’ depends on the concentrations of the acids and the time of the reaction. Cellulose trinitrate (12.2 – 13.2%N) is known as gun-cotton and is used in the manufacture of blasting explosives and smokeless powders.
(iv) Non-reducing sugar.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
