- Books Name
- A TEXT OF BIOLOGY - CLASS XII
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Biology
TISSUE CULTURE
Plant tissue culture is the technique of growing cells, tissue, organ or organism in sterilised nutrient media under controlled aseptic conditions.
Plant cells and organs can be cultured in vitro on a suitable medium. Haberlandt started the technique of plant tissue culture in 1902.
The method of producing thousands of plants through tissue culture is called clonal or micropropagation. Each of these plants will be identical (somaclones).
Culture medium can be liquid or solid. It contains source of carbon and energy (sucrose), minerals, glycine, vitamins, growth regulators (auxin like 2-4 D and cytokinin like BAP).
Plant part used for tissue culture is called explant. The explant and media are sterilized before culturing.
Explants are sterilized by specific antimicrobial chemicals (surface sterilization), while glassware and media can be sterilized by using steam and dry heat.
Callus Culture
A small piece of parenchymatous tissue is introduced over culture medium in a tube or flask in dark at 20° - 25°C.
The medium ordinarily contain the auxin, 2, 4 - D, and often a cytokinin like BAP.
After about 2-3 weeks it forms actively growing irregular and undifferentiated mass of cells called callus.
It is divided into several small sections and can be subcultured.
Each piece is then allowed to differentiate into plantlet by providing light and morphogenetic growth hormones.
Multiple Shoot Production :
It is used for raising numerous pathogen free copies of rare plants, hybrids and sterile plants.
A shoot tip or bud with 1 - 4 leaf primordia is sterilised and introduced over culture medium with high salt content and naphthalene acetic acid (NAA).
At intervals of 4 - 6 weeks the shoot tip is given cuts or shaken to form more buds. Each bud gives rise to a small plantlet.
Suspension Culture
In this technique, explant is suspended into liquid medium containing auxin, 2, 4 - D and is constantly agitated at the speed of 100 - 250 rpm (revolution per minute).
Agitation serves following three purposes :
(i) Aeration of culture
(ii) Constant mixing of medium
(ii) Breakage of cell aggregates into smaller groups.
Suspension cultures grow much faster than callus cultures.
In both the type of tissue cultures, with passage of time, cell/tissue dry matter increases and level of nutrient decreases.
To prevent the damage of newly formed cells, part of the cultures are regularly transferred to new culture vessels containing fresh media. This process is termed as subculturing.
Shoot Tip Culture or Production of Disease Free Plants :
Pathogen free clones of plants can be obtained through shoot-tip culture because shoot apical meristem is usually free of pathogens including virus due to high concentration of auxins and rapid rate of cell division.
The apical meristem accompanied by 1-2 leaf primordia is taken. For this the apical bud is sterilised.
The shoot tip is now placed over culture medium under aseptic conditions. Scientists have succeeded in culturing meristems of banana, sugarcane, potato.
Axillary meristem is also free of virus.
Somatic Embryo Regeneration :
Somatic embryos (embryoids) are embryos that arise from somatic cells in tissue culture.
The pattern of development of a somatic embryo proceed through globular, heart shaped, and torpedo shaped stages and mimic the development of sexually produced embryos.
Somatic embryo regeneration is induced by high concentration of auxin.
These embryos are also used to produce synthetic/ artificial seeds by encapsulating them in alginate.
Embryo Culture :
This involves excision of young embryo from seeds and their cultivation through tissue culture.
Embryo culture has following applications :
(i) In embryo rescue, interspecific hybrids are often sterile because of embryo mortality and seed collapse. In such cases the hybrid embryo is excised from female parent in early stage and is cultured. e.g., hybrid between common bean (Phaseolus vulgaris) and wild bean (P angustissimus).
(ii) Embryo culture allows seedling development in the plants whose seeds lack stored nutrients required for seedling growth. e.g., Orchid.
(iii) Also used in multiplication of some rare plants like makapuno coconuts.
Haploid Culture/Androgenic Haploid Culture/Pollen Grain Culture
This technique was developed by Guha and Maheshwari (1964) in Datura innoxia. The floral buds which are very young and unopened are first sterilized in clorox for 20-40 minutes.
They are then opened to remove anthers. Anthers are introduced over culture medium. Within 4-6 weeks, each anther gives rise to a number of haploid embryoids.
Normally they produce sterile haploid plants. Colchicine treatment results in chromosome doubling and produces homozygous diploids for each and every trait.
Gynogenic haploids are also possible by using unfertilized ovules.
Winter Wheat Jinghua-1 and Rice Guan-18 are two important varieties which are produced by this technique and are now under cultivation.
Uses of Androgenic Haploid
(i) Useful in mutation breeding
(ii) To maintain pure lines
(iii) To produce seedless varieties
Protoplast Fusion/Somatic Hybridisation/Parasexual Hybridisation
It is fusion of protoplasts of two plants belonging to different varieties, species and even genera. The cells are first treated with enzymes pectinase and cellulase.
These enzymes dissolve the cell wall and as a result naked protoplasts are produced.
The naked protoplasts are fused by electrofusion (high frequency alternating electric field with short current pulses) or chemofusion (through sodium nitrate or PEG = polyethyleneglycol).
It results in hybrid protoplasts.
The somatic hybrid may have a synkaryon (single fused nucleus) or heterokaryon (having two unfused nuclei).
The hybrid protoplast is called cytoplasmic hybrid or cybrid if one of the two nuclei of this get degenerated.
The first somatic hybrid was obtained by Carlson et.al. (1972) between Nicotiana glauca and N. langsdorfi (species of Tobacco).
The intergeneric somatic hybrids are Pomato (Potato × tomato) and Bomato (Brinjal and Tomato).
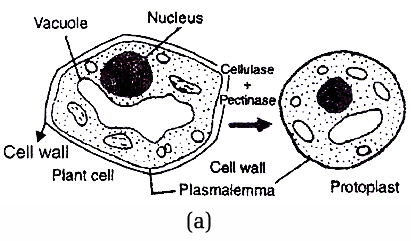
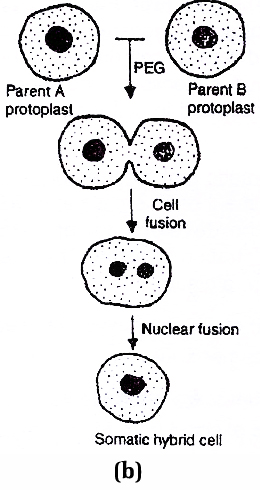
Fig. (a) Protoplast Preparation and (b) Fusion technique
Cellular Totipotency
It is ability of a plant cell to give rise to complete plant when cultured in a suitable culture medium at appropriate temperature and aeration conditions.
Each individual vegetative plant cell possess a complete genetic programme required to direct the development of an entire plant.
The term cellular totipotency was used for the first time by a German botanist Gottlieb Haberlandt (1902). He gave the idea that every plant cell is totipotent.
Applications of Tissue Culture
(i) Can be applied for crop improvement.
(ii) Can be applied for the rapid multiplication of desirable and rare plants.
(iii) Can be applied to obtain indefinite number of plants. (Help in micropropagation)
(iv) Can be applied to obtain virus free plants from shoot apex.
(v) Somaclonal Variations :
These variations are produced during tissue culture.
Some of these may be useful and stable e.g., better yield and quality, early maturation, resistance to diseases and pests, etc.
Some of the significant variations which have been taken up in plant breeding are, high protein content and resistance to late blight in Potato, increased shelf life in Tomato, resistance to rust and high temperature tolerance in Wheat, resistance to Tongro Virus and Leaf Hopper in Rice, short duration in Sugarcane etc.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
