Biotechnological applications in agriculture
- Books Name
- A TEXT OF BIOLOGY - CLASS XII
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Biology
BIOTECHNOLOGICAL APPLICATION IN AGRICULTURE
Food production can be enhanced by
(1) Agro-chemical based Agriculture
(2) Organic agriculture
(3) Genetically engineered crop-based agriculture
Green revolution resulted in increasing the food supply almost three times.
Green revolution is the great increase in the production of food grains (especially wheat and rice) that resulted in large part from the introduction of new, high yielding varieties begining in the mid 20th century.
Its early dramatic success was Maxico and the Indian subcontinent.
The new varieties required large amount of chemical fertilizers and pesticides to produce high yields, raising concern about cost and potentially harmful environmental effects.
This demands an alternate pathway that can result in maximum yield from the fields but the chemicals and fertiliser use is minimum i.e., harmful effects on the environment are reduced.
Genetically modified organisms or GMO can be the plants, bacteria, fungi and animals whose genes have been alteres by genetic manipulation.
(A) Genetically Modified Crops :
A transgenic crop is a crop that contains and expresses a transgene.
A popular term for transgenic crops is genetically modified crops or GM crops.
The techniques used for the production of transgenic crops offer the following two unique advantages: (i) any gene (from any organism or a gene synthesised chemically) can be used for transfer, and (ii) the change in genotype can be precisely controlled since only the transgene is added into the crop genome.
In contrast, breeding activities
(i) Can use only those genes that are present in such species that can be hybridised within them. In addition,
(ii) Changes occur in all those traits for which the parents used in hybridisation differ from each other.
When a transgene is introduced into the genome of an organism, it can achieve one of the following :
(i) Produces a protein that is the product in which we are interested.
(ii) Produces a protein that on its own produces the desired phenotype.
(iii) Modifies an existing biosynthetic pathway so that a new end-product is obtained.
(iv) Prevents the expression of an existing native gene.
Hirudin is a protein that prevents blood clotting. The gene enconding hirudin was chemically synthesised. This gene was then transferred into Brassica napus, where hirudin accumulates in seeds. The hirudin is purified and used as medicine. In this case, the transgene product itself is the product of interest.
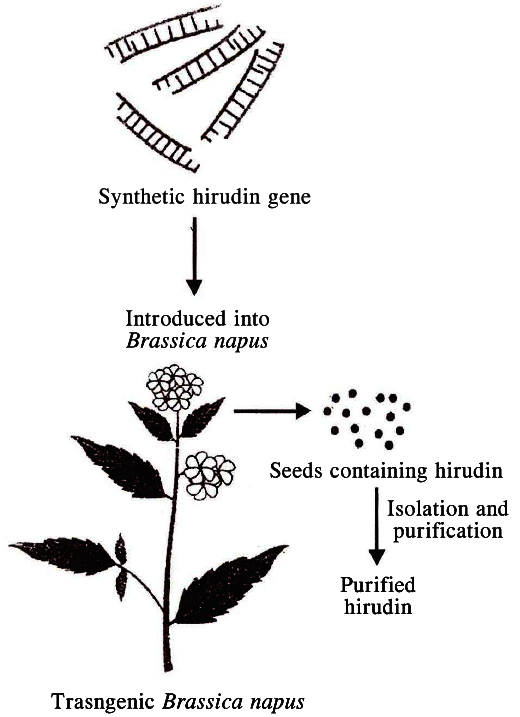
A simplified representation of the production of
hirudin from transgenic Brassica napus seeds
The tomato variety 'Flavr Savr' presents an example where expression of a native tomato gene has been blocked.
Expression of a native gene can be stopped by many different methods.
Fruit softening is promoted by the enzyme polygalacturonase which degrades pectin.
Production of polygalacturonase was blocked in, the transgenic tomato variety 'Flavr Savr'.
Therefore, fruits of this tomato variety remain fresh and retain their flavour much longer than do the fruits of normal tomato varieties. In addition, the fruits have a superior taste and increased total soluble solids these are unexpected bonus.
(B) Genetically Modified Food:
The food prepared from the produce of genetically modified (= transgenic) crops is called genetically modified food or, in short, GM food. GM food differs from the food prepared from the produce of conventionally developed varieties mainly in the following aspects.
Firstly, it contains the protein produced by the trans-gene in question, e.g., Cry protein in the case of insect resistant varieties.
Secondly, it contains the enzyme produced by the antibiotic resistance gene that was used during gene transfer by genetic engineering.
Finally, it contains the antibiotic resistance gene itself.
Concept Builder
Biofortification is a method of breeding crops to increase their nutritional value.
This can be done either through conventional selective breeding, or through genetic engineering.
Biofortification differs from ordinary fortification because it focuses on making plant foods more nutritious as the plants are growing, rather than having nutrients added to the foods when they are being processed.
This is an improvement on ordinary fortification when it comes to providing nutrients for the rural poor, who rarely have access to commercially fortified foods.
As such, biafortifications seen as an upcoming strategy for dealing with deficiencies of micronutrients in the developing world.
It has been argued that the above features of GM foods could lead to the following problems when they are consumed.
Firstly, the transgene product may cause toxicity and/or produce allergies.
Secondly, the enzyme produced by the antibiotic resistance gene could cause allergies, since it is a foreign protein.
Finally, the bacteria present in the alimentary canal of the human could takes up the antibiotic resistance gene that is present in the GM food.
These bacteria would then become resistant to the concerned antibiotic.
As a result, these bacteria could become difficult to manage.
The scientists involved in the production of transgenic crops are addressing to these concerns.
Efforts are being made to use other genes in place of antibiotic resistance genes.
The toxic and allergenic actions of the trans-gene product can be adequately examined by detailed assays using suitable animal models.
GM PRODUCTS: BENEFITS AND CONTROVERSIES
Benefits
(1) Crops
(i) Enhanced taste and quality
(ii) Reduced maturation time
(iii) Increased nutrients, yields, and stress tolerance
(iv) Improved resistance to disease, pests, and herbicides
(v) New products and growing techniques
(2) Animals
(i) Increased resistance, productivity, hardiness, and feed efficiency
(ii) Better yields of meat, eggs, and milk.
(iii) Improved animal health and diagnostic methods
(3) Environment
(i) "Friendly" bioherbicides and bioinsecticides
(ii) Conservation of soil, water, and energy
(iii) Bioprocessing for forestry prroducts
(iv) Better natural waste management
(v) More efficient processing
(4) Society
Increased food security for growing populations
Controversies
Safety: Potential human health impact: allergens, transfer of antibiotic resistance markers, unknown effects Potential environmental impact: unintended transfer of transgenes through cross-pollination, unknown effects on other organisms (e.g., soil microbes), and loss of flora and fauna biodiversity.
Bt COTTON
DNA technology makes it possible to locate the genes that produces Bt proteins lethal to insects and transfer the gene into crop plants.
First scientists identify a strain of Bt that kills the targeted insect.
Then they isolate the gene that produces the lethal protein.
That gene is removed from the Bt bacterium and a gene conferring resistance to a chemical (usually antibiotic or herbicide) is attached that proves useful in later steps.
The Bt gene with the resistance gene-attached is inserted into plant cells.
These modified or genetically transformed cells are then grown into complete plant by tissue culture.
The modified plant produces the same lethal protein as produced by the Bt bacteria because plants now have the same gene.
Concept Builder
B. thuringiensis was first discovered in 1902 by Japanese biologist Shigetane Ishwatari.
In 1911, B. thuringiensis was rediscovered in Germany by Ernst Berliner, who isolated it as the cause of a disease called Schlaffsucht in flour moth caterpillars.
In 1976, Zakharyan reported the presence of a plasmid in a strain of B. thuringiensis and suggested the plasmid's involvement in endospore and crystal formation.
B. thuringiensis is closely related to B.cereus, a soil bacterium, and B.anthracis, the cause of anthrax: the three organisms differ mainly in their plasmids.
Like other members of the genus, all three are aerobes capable of producing endospores. Upon sporulation, B. thuringiensis forms crystals of proteinaceous insecticidal -endotoxin (called crystal proteins of Cry proteins), which are encoded by cry genes.
In most strains of B. thuringiensis the cry genes are located on the plasmid.
Cry toxins have specific activities against insect species of the orders Lepidoptera (moths and butterflies), diptera (flies and mosquitoes), coleoptera (beetles), hymenoptera (wasps, bees, ants and sawflies) and nematodes.
Thus, B. thurengiensis serves as an important reservoir of Cry toxins for production of biological insecticides and insect-resistant genetically modified crops.
When insects ingest toxin crystals, the alkaline pH of their digestive tract activates the toxin.
Cry inserts into the insect gut cell membrane, forming a pore. The pore results cell lysis and eventual death of the insect.
B. thuringiensis forms protein crystals during a particular phase of their growth.
These crystals contain a toxic insecticidal protein.
Why does this toxin not kill the Bacillus? Actually, the Bt toxin protein exists as inactive protoxin but once an insect ingests the inactive toxin, it is converted into an active form of toxin due to the alkaline pH of the gut which solubilises the crystals.
The activated toxin binds to the surface of midgut epithelial cells and creates pores that cause cell swelling and lysis and eventually cause death of the insect.
Bt is not harmful to humans, other mammals, birds, fish or beneficial insects.
Specific Bt toxin genes were isolated from Bacillus thuringiensis and incorporated into the several crop plants such as cotton.
The choice of genes depends upon the crop and the targeted pest, as most Bt toxins are insect-group specific.
The toxin is coded by a gene named cry. There are a number of them, for example, the proteins encoded by the genec cry I Ac and cry II Ab control the cotton bollworm, that of cry I Ab controls corn borer.
Although Bt genes have been introduced into tobacco, tomatoes, cotton, and other broadleaf Plants, gene transfer technology for corn is a recent achievement.
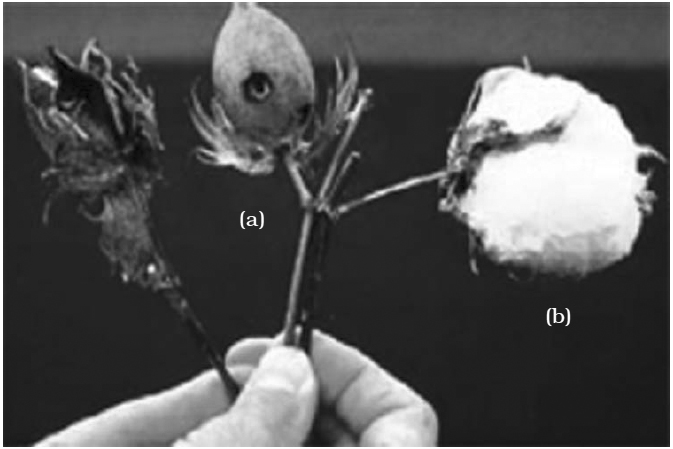
The development of corn plants expressing Bt proteins requires substantial changes in the Bt genes, including the creation of synthetic versions of the genes, rather than the microbial Bt gene itself.
Concept Builder
There are several advantages in expressing Bt toxins in transgenic Bt crops:
The level of toxin expression can be very high thus delivering sufficient dosage to the pest:
The toxin expression is contained within the plant system and hence only those insects that feed on the crop perish.
The toxin expression can be modulated by using tissue-specific promoters, and replaces the use synthetic pesticides in the environment: The latter observation has been well documented Worldwide.
PEST RESISTANT PLANTS
Root-knot nematodes are the most economically important group of plant-parasitic nematodes worldwide. They attack nearly every food and fiber crop grown, about 2,000 plant species in all.
The nematode invades plant roots, and by feeding on the roots' cells, they cause the roots to grow large form galls, or knots, damaging the crop and reducing its yields .
The most cost-effective and sustainable management tactic for preventing root-knot nematode damage and reducing growers' losses is to develop resistant plants that prevent the nematode from feeding on the roots.
Because root-knot nematode resistance doesn't come naturally in most crops, bioengineering is required.
Four common root-knot nematode species (mainly Meloidegyne incognitia) account for 95 percent of all infestations in agricultural land.
By discovering a root-knot nematode parasitism gene that's essential for the nematode to infect crops, the scientists have developed a resistance gene effective against all four species.
Using a technique called RNA interference (RNAi), the researchers have effectively turned the nematode's biology against itself.
They genetically modified Arabidopsis, a model plant, to produce double-stranded RNA (dsRNA) to knock out the specific parasitism gene in the nematode when it feeds on the plant roots.
Concept Builder
Long double-stranded RNAs (dsRNAs; typically > 200nt) can be used to silence the expression of target genes in a variety of organisms and cell types (e.g.; worms, fruit flies and plants).
Upon introduction, the long dsRNAs enter a cellular pathway that is commonly referred to as the RNA interference (RNAi) pathway.
First, the dsRNA get processed into 20-25 nucleotide (nt) small interfering RNAs (siRNAs) by an RNase III-like enzyme called Dicer (initiation step).
Then, the complexes (RISCs), unwinding in the process.
The siRNA strands subsequently guide the RISCs to complementary RNA molecules, where they cleave and destroy the cognate RNA (effecter step).
Cleavage of cognate RNA takes place near the middle of the region bound by the siRNA strand.
In mammalian cells, introduction of long dsRNA (>30 nt) initiates a potent antiviral response, exemplified by nonspecific inhibition of protein synthesis and RNA degradation.
The mammalian antiviral response can be bypassed. however, by the introduction or expression of siRNAs.
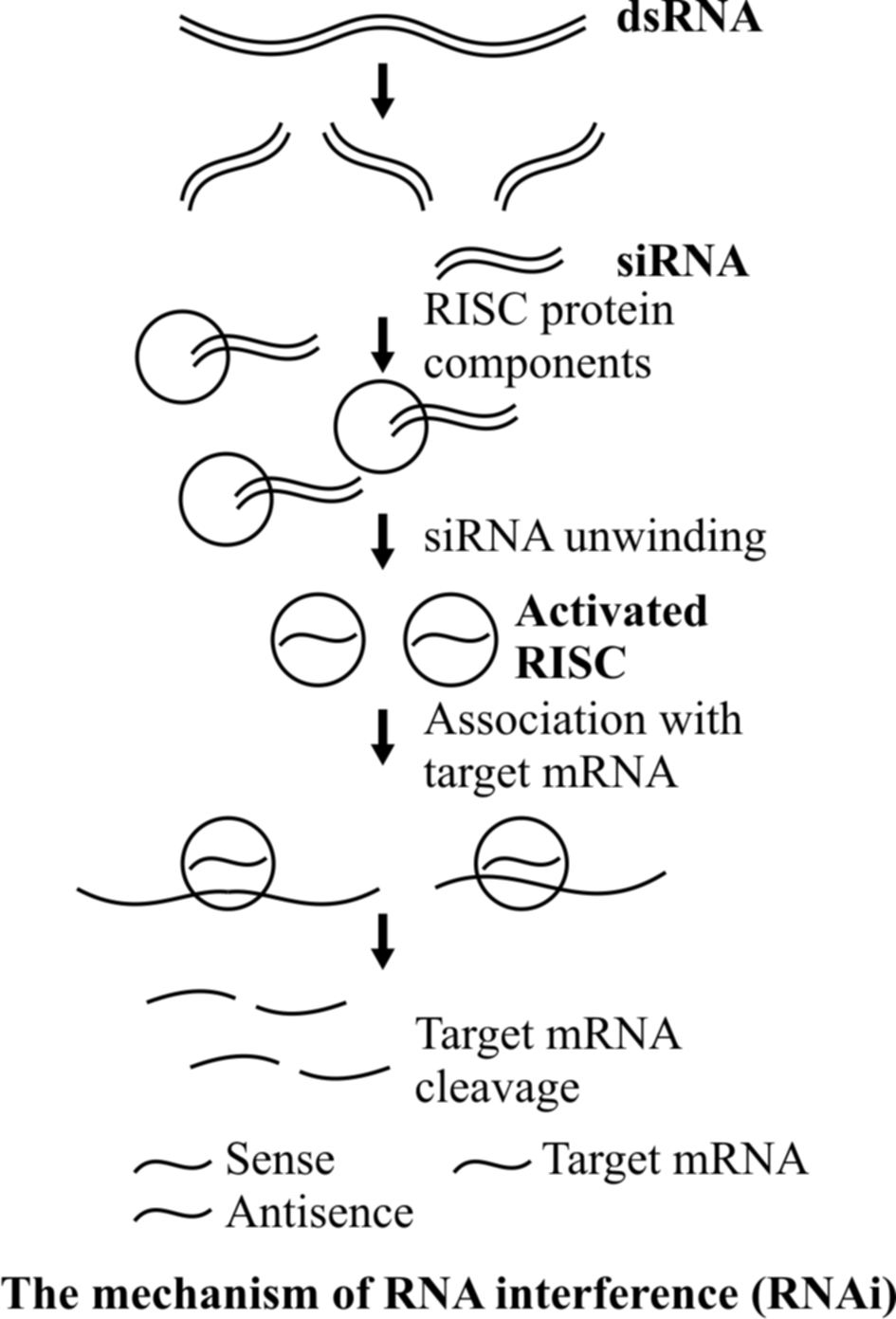
RNAi takes place in all eukaryotic organisms as a method of cellular defense.
This method involves silencing of a specific mRNA due to a complementary dsRNA molecule that binds to and prevents translation of the mRNA (silencing).
The source of this complementary RNA could be from an infection by viruses having RNA genomes or mobile genetic elements (transposons) that replicate via an RNA intermediate.
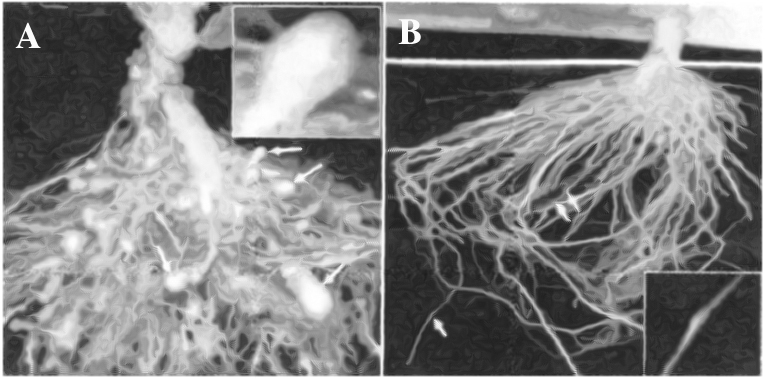
(A) Roots of a typical control plant (B) Transgenic plant roots 5 days after deliberate
infection of nematode but protected through novel mechanism.
Using Agrobacterium vectors, nematode-specific genes were introduced into the host plant.
The introduction of DNA was such that it produced both sense and anti-sense RNA in the host cells.
These two RNAs being complementary to each other formed a double stranded RNA that initiated RNAi and thus, silenced the specific mRNA of the nematode.
The consequence was that the parasite could not survive in a transgenic host expressing specific interfering RNA.
The transgenic plant therefore got itself protected from the parasite.
This knocked out the parasitism gene in the nematode and disrupted its ability to infect plants.
"No natural root-knot resistance gene has this effective range of root-knot nematode resistance."
The efforts have been directed primarily at understanding the molecular tools the nematode uses to infect plants.
This is a prerequisite for bioengineering durable resistance to these nematodes in crop plants.
Biotechnological applications in medicine
- Books Name
- A TEXT OF BIOLOGY - CLASS XII
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Biology
BIOTECHNOLOGICAL APPLICATION IN MEDICINE
1. Therapeutic agents
Proteins with potential as pharmaceutical agents are produced by using genetically engineered organisms.
Enzymes have also been used for this purpose, e.g., DNase I and alginate lyase have been used in aerosols.
Some known examples are given below:
1. Human growth hormone obtained from E. coli is used for treatment of dwarfness.
2. Chorionic gonadotropin hormone produced by genetic engineering is used for treatment of infertility.
3. Interferons produced by E. coli are commercially used for treatment of viral infections and cancer. Interferons were first obtained through DNA recombinant technique by Charles Weisman in 1980. He inserted the gene for interferon production in E. coli.
4. Interleukins produced by E. coli are used for stimulating immunity system.
5. Tissue Plasminogen Activator (TPA) -an enzyme is used for dissolving blood clot after heart attack and stroke.
6. Antihemophilic human factor VIII is used by people with hemophilia to prevent and control bleeding or to prepare them for surgery.
7. Platelet derived growth factor produced by recombinant DNA technology is useful for stimulating wound healing.
8. Penicillin G acylase is also produced by genetic engineering. This enzyme is used for converting penicillin into 6-amino penicilline acid for the formation of new antibiotics.
2. Genetically engineered insulin
Since the discovery of insulin by Banting and Best (1921), and its use for the treatment of diabetes, it was derived from pancreatic glands of abattoir animals.
This hormone, produced and secreted by the beta cells of the pancreas islets of Langerhans, regulates the use and storage of food, particularly carbohydrates.
Although bovine and porcine insulin is similar to human insulin, their composition is slightly different.
It, therefore, causes adverse effects due to regular injection, this being a foreign substance.
This observation led to the synthesis of human insulin which is chemically identical to its naturally produced component.
Insulin consists of 51 amino acids forming two short polypeptide chains-chain A having 21 amino acids and chain B with 30 amino acids.
The two chains are linked by disulfide bond. In animals, including humans, insulin occurs as proinsulin.
It is made of chain A, chain B and chain C (30 amino acids). As the insulin matures, chain C is removed.
The genetic engineering of insulin begins with identification and separation of DNA sequences coding for chain A and chain B.
This was found to be present at the top of the short arm of the eleventh chromosome.
It contains 153 nucleotides-63 nucleotides for chain A and 90 nucleotides for chain B.
These sequences were introduced into plasmid (pBR322) of Escherichia coli -common human colon bacterium.
It is said to be the factory used in genetic engineering of insulin.
In E. coli, -galactosidase controls the transcription of these genes, therefore, insulin gene needs to be tied to this enzyme.
The protein formed by E. coli consists partly of -galactosidase joined to either A or B chain of insulin.
These are then extracted from -galactosidase fragment and purified.
The two chains are mixed and reconnected in a reaction that forms disulfide bridges resulting in pure humulin-the synthetic human insulin.
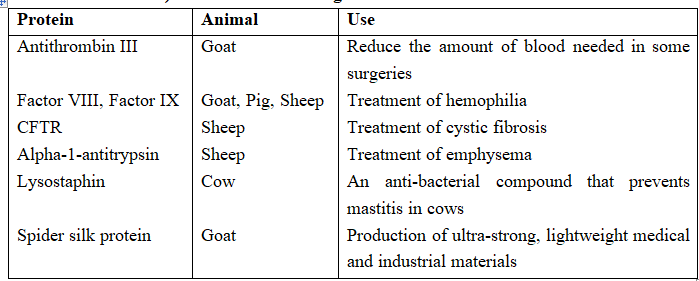
Proteins with Therapeutic and Industrial Value that have been Produced (but not Commercialized) in the Milk of Transgenic Animals
GENE THERAPY
Much attention has been focussed on the so-called genetic metabolic diseases in which a defective gene causes an enzyme to be either absent or ineffective in catalyzing a particular metabolic reaction effectively.
A potential approach to the treatment of genetic disorders in man is gene therapy.
This is a technique whereby the absent or faulty gene is replaced by a working gene, so that the body can make the correct enzyme or protein and consequently eliminate the root cause of the disease.
The first clinical gene therapy was given in 1990 to a 4-year old girl with adenosine deaminase (ADA) deficiency.
This enzyme is crucial for the immune system to function.
The disorder is caused due to the deletion of the gene for adenosine deaminase.
In some children, ADA deficiency can be cured by bone marrow transplantation; in others, it can be treated by enzyme replacement therapy, in which functional ADA is given to the patient by injection.
But the problem with both of these approaches is that they are not completely curative.
As a first step towards gene therapy, lymphocytes from the blood of the patient are grown in a culture outside the body.
A functional ADA cDNA (using a retroviral vector) is then introduced into these lymphocytes, which are subsequently returned to the patient.
However, as these cells are not immortal, the patient requires periodic infusion of such genetically engineered lymphocytes.
However, if the gene isolated from bone marrow cells producing ADA is introduced into cells at early embryonic stages, it could be a permanent cure.
Before treatment for a genetic disease can begin, an accurate diagnosis of the genetic defect needs to be made.
It is here that biotechnology is also likely to have a great impact in the near future.
Genetic engineering research has produced a powerful tool for pinpointing specific diseases rapidly and accurately.
Short pieces of DNA called DNA probes can be designed to stick very specifically to certain other pieces of DNA.
The technique relies upon the fact that complementary pieces of DNA stick together.
DNA probes are more specific and have the potential to be more sensitive than conventional diagnostic methods, and it should be possible in the near future to distinguish between defective genes and their normal counterparts, an important development.
Molecular Diagnosis
For effective treatment of a disease, early diagnosis and understanding its pathophysiology is very important.
Using conventional methods of diagnosis (serum and urine analysis, etc.), early detection is not possible.
Recombinant DNA Technology, Polymerase Chain Reaction (PCR) and Enzyme Linked Immuno-Sorbent Assay (ELISA) are some of the techniques that serve the purpose of early diagnosis.
Presence of a pathogen (bacteria, viruses, etc.) is normally suspected only when the pathogen has produced a disease symptom.
By this time the concentration of pathogen is already very high in the body.
However, very low concentration of a bacteria or virus (at a time when the symptoms of the disease are not yet visible) can be detected by amplification of their nucleic acid by PCR, which is now routinely used to detect HIV in suspected AIDS patients.
It is being used to detect mutations in genes in suspected cancer patients too.
It is a powerful technique to identify many other genetic disorders.
DNA is usually isolated from White blood cells & has to be cut into smaller pieces to be analysed.
This is accomplished by restriction enzymes. Eco RI (a restriction enzyme from E. coli) will cut DNA wherever the sequence GAATTC appears.
Exposure to this enzyme results in the DNA being chopped into millions of fragments of varying size, called restriction fragments.
Once cut, the DNA is loaded into a well on one end of a slab of gel.
The fragments are then separated according to size by electrophoresis.
As electric current passes through the gel, the fragments move according to size.
The bigger fragments stay close to the origin, and the smaller fragments move farther down the length of the gel.
The DNA is then denatured (by exposure to alkaline solutions) to render the DNA single stranded (instead of the natural double-stranded form).
Since the gel is difficult to handle, the DNA is transferred to a nitro cellulose paper to create a Southern blot (named after the researcher who developed the procedure).
The DNA probe which is radioactively labeled (or fluorescently labeled) is then applied to the Southern blot.
Since the probe is also single-stranded, it will seek the single-stranded DNA fragments that are complementary, and undergo hybridization.
The excess probe is washed out and only the bound probe will remain on the Southern blot paper.
This is then laid on an X-ray film.
The radioactive probe will leave bands on the X-ray film.
Depending on the type of probe used, there could be hundreds of bands (much like bar codes) or only a few bands present on the X-ray film.
By having several wells on the end of the gel, several samples can be loaded, and DNA fragments in corresponding lanes can be analyzed concurrently.
By running control samples, with known DNA fragment sizes, on the same gel with patient samples, it is possible to identify changes in the size of a DNA fragment and, therefore, a change in a specific gene.
Since each step takes about a day and since samples are batched, the procedure ordinarily takes one to two weeks to complete.
ELISA is based on the principle of antigen-antibody interaction. Infection by pathogen can be detected by the presence of antigens (proteins, glycoproteins, etc.) or by detecting the antibodies synthesised against the pathogen.
Transgenic Animals
- Books Name
- A TEXT OF BIOLOGY - CLASS XII
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Biology
Transgenic Animal
There are various definitions for the term transgenic animal.
A transgenic animal is one whose genome has been changed to carry genes from other species.
The nucleus of all cells in every living organism contains genes made up of DNA.
These genes store information that regulates how our bodies form and function.
Genes can be altered artificially, so that some characteristics of an animal are changed.
For example, an embryo can have an extra, functioning gene from another source artificially introduced into it, or a gene introduced which can knock out the functioning of another particular gene in the embryo.
Animals that have their DNA manipulated in this way are known as transgenic animals.
The majority of transgenic animals produced so far are mice, the animal that pioneered the technology.
The first successful transgenic animal was a mouse. A few years later, it was followed by rabbits, pigs, sheep, and cattle.
How are transgenic animals produced?
To date, there are three basic methods of producing transgenic animals:
(i) DNA microinjection
(ii) Retrovirus-mediated gene transfer
(iii) Embryonic stem cell-mediated gene transfer
Gene transfer by microinjection is the predominant method used to produce transgenic farm animals.
Since the insertion of DNA results in a random process, transgenic animals are mated to ensure that their offsprings acquire the desired transgene.
However, the success rate of producing transgenic animals individually by these methods is very low and it may be more efficient to use cloning techniques to increase their numbers.
For example, gene transfer studies revealed that only 0.6% of transgenic pigs were born with a desired gene after 7,000 eggs were injected with a specific transgene.
How do transgenic animals contribute to human welfare?
The benefits of these animals to human welfare can be grouped into following areas:
(1) Agriculture (2) Medicine (3) Industry
The examples below are not intended to be complete but only to provide a sampling of the benefits.
1. Agricultural Applications
(a) Breeding: Farmers have always used selective breeding to produce animals that exhibit desired traits (e.g., increased milk production, high growth rate). Traditional breeding is a time-consuming, difficult task. When technology using molecular biology was developed, it became possible to develop traits in animals in a shorter time and with more precision. In addition, it offers the farmer an easy way to increase yields.
(b) Quality: Transgenic cows exist that produce more milk or milk with less lactose or cholesterol, pigs and cattle that have more meat on them, and sheep that grow more wool. In the past, farmers used growth hormones to spur the development of animals but this technique was problematic, especially since residue of the hormones remained in the animal product.
(c) Disease resistance : Scientists are attempting to produce disease-resistant animals, such as influenza-resistant pigs, but a very limited number of genes are currently known to be responsible for resistance to diseases in farm animals.
2. Medical Applications
(a) Xenotransplantation : Patients die every year for lack of a replacement heart, liver, or kidney. For example, about 5,000 organs are needed each year in the United Kingdom alone. Transgenic pigs may provide the transplant organs needed to alleviate the shortfall. Currently, xenotransplantation is hampered by a pig protein that can cause donor rejection but research is underway to remove the pig protein and replace it with a human protein.
(b) Nutritional supplements and pharmaceuticals: Products such as insulin, growth hormone, and blood anti-clotting factors may soon be or have already been obtained from the milk of transgenic cows, sheep, or goats. Research is also underway to manufacture milk through transgenics for treatment of debilitating diseases such as phenylketonuria (PKU), hereditary emphysema, and cystic fibrosis.
In 1997, the first transgenic cow, Rosie, produced human protein-enriched milk at 2.4 grams per litre. This transgenic milk is a more nutritionally balanced product than natural bovine milk and could be given to babies or the elderly with special nutritional or digestive needs. Rosie's milk contains the human gene -lactalbumin.
(c) Vaccine safety: Transgenic mice are being developed for use in testing the safety of vaccines before they are used on humans. Transgenic mice are being used to test the safety of the polio vaccine. If successful and found to be reliable, they could replace the use of monkeys to test the safety of batches of the vaccine.
3. Industrial Applications
In 2001, two scientists at Nexia Biotechnologies in Canada spliced spider genes into the cells of lactating goats.
The goats began to manufacture silk along with their milk and secrete tiny silk strands from their body by the bucketful.
By extracting polymer strands from the milk and weaving them into thread, the scientists can create a light, tough, flexible material that could be used in such applications as military uniforms, medical microsutures, and tennis racket strings.
Toxicity-sensitive transgenic animals have been produced for chemical safety testing.
Microorganisms have been engineered to produce a wide variety of proteins, which in turn can produce enzymes that can speed up industrial chemical reactions.
The anthrax bacterium sent through letters after September 2001.
Mass-produced pathogens or their toxins are delivered either as powder or in the form of spray, using a variety of delivery devices.
Bioweapons (a) are low-cost weapons, (b) cause for more casualities than chemical or conventional weapons, and (c) bioweapon agents are invisible, and extremely difficult to detect.
These features make bioweapon agents very convenient for use by terrorists and even governments, and both have used them on a limited scale.
The possible defences against bioweapons include the use of respirator or gas mask, vaccination, administration of appropriate antibiotics, and decontamination. In addition, sensitive detection systems should be develped to control and minimise damage.
Ethical Issues
- Books Name
- A TEXT OF BIOLOGY - CLASS XII
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Biology
BIOETHICS
Ethics includes a set of standards by which a community regulates its behaviour and decides as to which activity is legitimate and which is not.
Therefore, bioethics may be viewed as a set of standards that may be used to regulate our activities in relation to the biological world.
Biotechnology, particularly recombinant DNA technology, is focussed on' exploiting the biological world in ways that are usually unprecedented.
Therefore, biotechnology has been labelled variously, ranging from 'unnatural' to 'detremental, to 'biodiversity'.
The major bioethical concerns pertaining to biotechnology are summarised below:
(i) Use of animals in biotechnology causes great suffering to them.
(ii) When animals are used for production of pharmaceutical proteins, they are virtually reduced to the status of a 'factory'.
(iii) Introduction of a transgene from one species into another species violates the 'integrity of species'.
(iv) Transfer of human genes into animals (and vice-versa ) dilutes the concept of 'humanness'.
(v) Biotechnology is disrespectful to living beings, and only exploits them for the benefit of human beings.
(vi) Biotechnology may pose unforeseen risks to the environment, including risk to biodiversity.
These arguments may seem quite attractive.
It may be pointed out that biotechnology usually does only what was being done before.
However, biotechnologies do these things on a much larger scale and at a much faster rate.
Each society has to evaluate for itself the validity of these and other arguments related to biotechnology.
It also has to decide the kinds of activities that it considers acceptable, and those that it does not.
Going beyond the morality of such issues, the biological Significance of such things is also important.
Genetic modification of organisms can have unpredictable results when such organisms are introduced into the ecosystem.
Therefore, the Indian Government has set up organisations such as GEAC (Genetic Engineering Approval Committee), which will make decisions regarding the validity of GM research and the safety of introducing GM-organisms for public services.
The modification/usage of living organisms for public services (as food and medicine sources, for example) has also created problems with patents granted for the same.
BIOPATENT
A patent is the right granted by a government to an inventor to prevent others from commercial use of his invention.
A patent is granted for (a) an invention (including a product), (b) an improvement in an earlier invention, (c) the process of generating a product, and (d) a concept or design.
Initially, patents were granted for industrial inventions, etc.
But at present, patents are being granted for biological entities and for products derived from them ; these patents are called biopatents.
Primarily, industrialised countries, like U.S.A., Japan and members of European Union, are awarding Biopatents.
Biopatents are awarded for the following : (i) strains of microorganisms, (ii) cell lines, (iii) genetically modified strains of plants and animals, (iv) DNA sequences, (v) the proteins encoded by DNA sequences, (vi) various biotechnological procedures, (vii) production processes, (viii) products, and (ix) product applications.
There has been a great deal of opposition from various social groups to the patenting of life forms.
The nature of these objections is mainly ethical and political.
The arguments in favour of biopatents are primarily of increased economic growth.
Concept Builder
Intellectual property (IP) is a term referring to a number of distinct types of creation of the mind for which a set of exclusive rights are recognized and the corresponding fields of law.
Under intellectual property law, owners are granted certain exclusive rights to a variety of intangible assets, such as musical, literary, and artistic works; discoveries and inventions; and words, phrases, symbols and designs.
Common types of intellectual property include copyrights, trademarks, patents, industrial design rights and trade secrets in some jurisdictions.
Many biotechnology patents are very broad in their coverage.
For example, one patent covers 'all transgenic plants of Brassica family'.
Such broad patents are considered .morally unacceptable and fundamentally inequitable, since these would enable financially powerful corporations to acquire monopoly control over biotechnological processes.
They may, in the end, even come to control the direction of agricultural research, including plant breeding.
Such a position would pose a threat to the global food security.
Any organisations and multinational companies exploit and/or patent biological resources or bioresources of other nations without proper authorisation from the countries concerned; this is known as biopiracy.
The industrialised nations are rich in technology and financial resources but poor in biodiversity and traditional knowledge related to the utilisation of the bioresources.
In contrast, developing nations are poor in technology and financial resources, but are rich in biodiversity and traditional knowledge related to bioresources.
Biological resources or bioresources include all those organisms that can be used to derive commercial benefits.
Traditional knowledge related to bioresources is the knowledge developed by various communities over long periods of history, regarding the utilisation of the bioresources, e.g., use of herbs, etc. as drugs.
Often, this traditional knowledge can be exploited to develop modern commercial processes.
The traditional knowledge suggests the direction to be followed, and saves considerable time, effort and expenditure for their commercialisation.
Institutions and companies of industrialised nations are collecting and exploiting the bioresources, as follows,
(i) They are collecting and patenting the genetic resources themselves. For example, a patent granted in U.S.A covers the entire 'basmati' rice germplasm indigenous to our country.
(ii) The bioresources are being analysed for identification of valuable biomolecules. A biomolecule is a compound produced by a living organism. The biomolecules are then patented and used for commercial activities.
(iii) Useful genes are isolated from the bioresources and patented. These genes are then used to generate commercial products.
(iv) The traditional knowledge related to bioresources is utilised to achieve the above objectives. In some cases, the traditional knowledge itself may be the subject of a patent.
A west African plant, Pentadiplandra brazzeana produces a protein called brazzein, which is approximately 2,000 times as sweet as sugar.
In addition, brazzein is a low-calorie sweetener.
Local people have known and used the super-sweet berries of this plant for centuries.
But the protein brazzein was patented in U.S.A.
Subsequently, the gene encoding brazzein was also isolated, sequenced and patented in U.S.A.
It is proposed to transfer the brazzein gene into maize and express it in maize kernels.
These kernels will then be used for the extraction of brazzein.
This development could have serious implications for countries exporting large quantities of sugar.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
