Liquid Surfaces
Certain properties of free surfaces:-
Whenever liquids are poured in any container they take the shape of that container in which they are poured and they acquire a free surface.
Consider a case if we pour water inside the glass it takes the shape of the glass with a free surface at the top.
Top surface of the glass is a free surface. Water is not in contact with anything else, it is in contact with the air only. This is known as free surfaces. Liquids have free surfaces. As liquids don’t have a fixed shape they have only fixed volume.
Free surfaces have additional energy as compared to inner surfaces of the liquid called surface energy.

Surface Energy
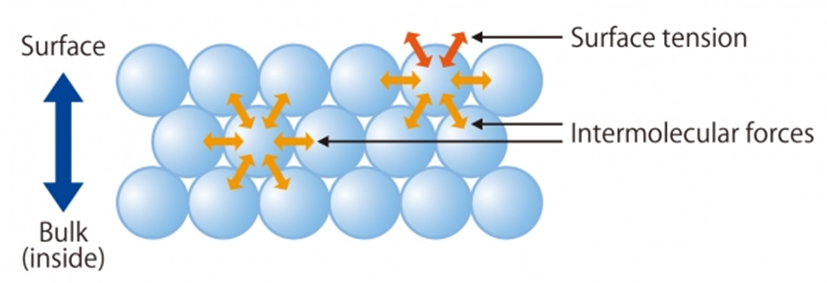
Surface energy is the excess energy exhibited by the liquid molecules on the surface compared to those inside the liquid. This means liquid molecules at the surface have greater energy as compared to molecules inside it.
Suppose there is a tumbler and when we pour water in the tumbler, it takes the shape of the tumbler. It acquires a free surface.
Case 1: When molecules are inside the liquid:-
Suppose there is a molecule inside the water, there will be several other molecules that will attract that molecule in all directions.
As a result, this attraction will bind all the molecules together. This results in negative potential energy of the molecule as it binds the molecule.
To separate this molecule a huge amount of energy is required to overcome potential energy. Some external energy is required to move this molecule and it should be greater than the potential energy.
Therefore a large amount of energy is required by the molecules which are inside the liquid.
Case2: When the molecules are at the surface:-
When the molecule is at the surface, half of it will be inside and half of it is exposed to the atmosphere.
The lower half of the molecule, it will be attracted by the other molecules inside the liquid. But the upper half is free. The negative potential energy is only because of the lower half.
But the magnitude is half as compared to the potential energy of the molecule which is fully inside the liquid. So the molecule has some excess energy, because of this additional energy which the molecules have at the surface different phenomena happen like surface energy, and surface tension.
Liquids always tend to have the least surface area when left to themselves.
As more surface area will require more energy as a result liquids tend to have less surface area.
Surface Tension
Surface tension is the property of the liquid surface which arises due to the fact that surface molecules have extra energy.
Surface tension is the surface energy per unit area of the liquid surface. It can be also defined as Force per unit length on the liquid surface
Surface tension(S) =Surface Energy/area
At any interface (it is a line that separates two different mediums) the surface tension always acts in the equal and opposite directions and it is always perpendicular to the line at the interface.
A fluid will stick to a solid surface if the surface energy between fluid and solid is smaller than the sum of energies between solid-air and fluid-air.
This means Ssf (solid-fluid) < Sfa (fluid air) + Ssa (Solid air)
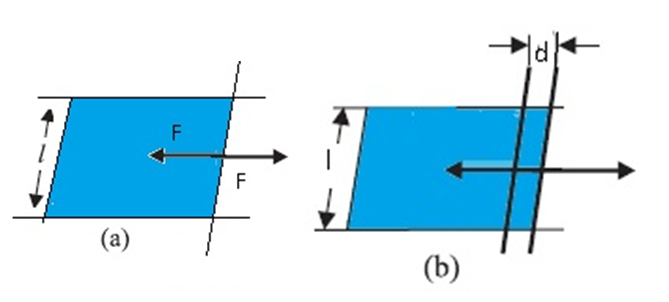
Stretching a film (a) A film in equilibrium ;(b) the film stretched an extra distance.
Why does water stick to glass but Mercury doesn’t?
In the case of water and glass, water sticks to glass because the surface energy of water and glass is less than the surface energy between water and air and between glass and air. S.E (w-g) < S.E (w-a) +S.E (g-a)
In the case of mercury, Surface Energy between mercury and glass S.E (m-g), Surface energy between mercury and air S.E (m-a), and Surface Energy between air and glass S.E (a-g). E (m-g)> S.E (m-a) +S.E (a-g)

Angle of Contact
The angle of contact is the angle at which a liquid interface meets a solid surface. It is denoted by θ. It is different at the interfaces of different pairs of liquids and solids.
For example - A droplet of water on a lotus leaf. The droplet of water (Liquid) is in contact with the solid surface which is a leaf.
This liquid surface makes some angle with the solid surface. This angle is known as the angle of contact.

Water forms a spherical shape on the lotus leaf but it splits on the table.
Significance of Angle of Contact
Angle of contact determines whether a liquid will spread on the surface of a solid or it will form droplets on it.
- If the Angle of contact is obtuse: then droplets will be formed.
- If the Angle of contact is acute: then the water will spread.
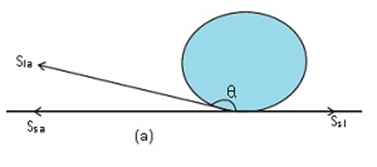
Case1: When the droplet is formed
Consider we have a solid surface, a droplet of water which is liquid and air.
The solid-liquid interface is denoted by Ssl, the solid air interface is denoted by Ssa and the liquid-air interface is denoted by Sla.
The angle which Ssl makes with Sla. It is greater than 90 degrees. Therefore a droplet is formed.
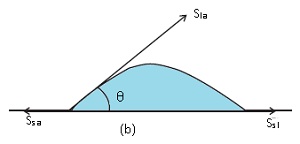
Case 2: When water just spreads
The angle at which liquid forms with a solid surface is less than 90 degrees.
Drops and Bubbles
Why are water and bubbles drop?
Whenever liquid is left to itself it tends to acquire the least possible surface area so that it has the least surface energy so it has the most stability.
Therefore for more stability, they acquire the shape of a sphere, as the sphere has the least possible area.

Spherical Shape
Distinction between Drop, Cavity and Bubble
- Drop: - Drop is a spherical structure filled with water.
- There is only one interface in the drop.
- The interface separates water and air.

Water droplets
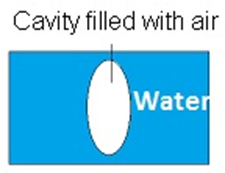
Cavity: -Cavity is a spherical shape filled with air. In the surroundings there is water and in the middle, there is a cavity filled with air. There is only one interface that separates air and water.
Example: - bubble inside the aquarium.
Bubble: - In a bubble there are two interfaces. One is air and water and another is water and air. Inside a bubble there is air and there is air outside. But it consists of a thin film of water.

Pressure inside a drop and a cavity
Pressure inside a drop is greater than the pressure outside.
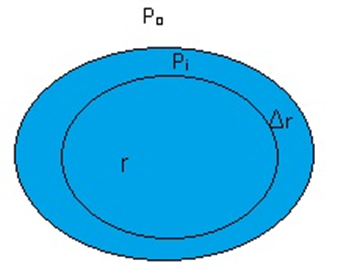
Suppose there is a spherical drop of water of radius ‘r’ which is in equilibrium.
Consider there is an increase in radius which is Δr.
Therefore Extra Surface energy = Surface Tension(S) x area
Sla x 4π(r+Δr) 2 – Slax4πr2
After calculating Extra Surface energy=8πr Δr Sla
At Equilibrium, Extra Surface energy = Energy gain due to the pressure difference
8πr Δr Sla = (Pi - Po) 4πr2xΔr
Where Pi= Pressure inside the drop and Po = Pressure outside the drop.
After calculation Pi - Po = 2 Sla/r
Pressure inside a Bubble
- Pressure inside a bubble is greater than the pressure outside.
- As bubble has 2 interfaces, Pi-Po=2Sla/r x 2
- Therefore, Pi-Po=4Sla/r

Conclusion: - In general, for a liquid-gas interface, the convex side has a higher pressure than the concave side.

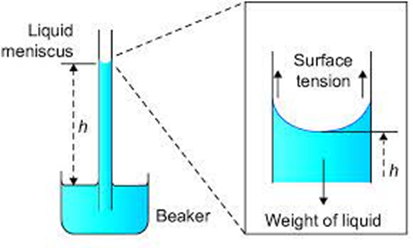
Capillary Rise
In Latin the word Capilla means hair. Due to the pressure difference across a curved liquid-air interface, the water rises up in a narrow tube in spite of gravity.
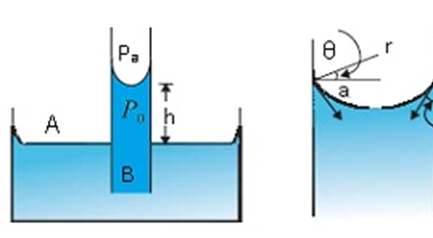
Consider a vertical capillary tube of circular cross-section (radius a) inserted into an open vessel of water.
The contact angle between water and glass is acute. Thus the surface of the water in the capillary is concave. As a result, there is a pressure difference between the two sides of the top surface. This is given by
(Pi – Po) = (2S/r) = 2S/ (a sec θ) = (2S/a) cos θ (i)
Thus the pressure of the water inside the tube, just at the meniscus (air-water interface) is less than the atmospheric pressure.
Consider the two points A and B. They must be at the same pressure,
P0 + h ρ g = Pi = PA (ii)
Where ρ is the density of water, and h is called the capillary
h ρ g = (Pi – P0) = (2S cos θ)/a (By using equations (i) and (ii))
Therefore the capillary rise is due to surface tension. It is larger, for a smaller radius.

 Madhava Publications
Madhava Publications
