Introduction
In this chapter, we will study some of the thermal properties of matter.
This topic discusses various thermal phenomena and how a matter behaves when subjected to the flow of thermal energy. We are specifically concerned in
- Thermal expansion
- Heat and calorimetry
- Transfer of heat
We all have common-sense notions of heat and temperature. Temperature is a measure of ‘hotness’ of a body. A kettle with boiling water is hotter than a box containing ice. In physics, we need to define the notion of heat, temperature, etc., more carefully.
When the body is heated, various changes take place. It could expand, it can become hotter, it can change phase etc. Temperature is a measure of the hotness of a body. When water boils or freezes, its temperature does not change during these processes even though a great amount of heat is flowing into or out of it.
You might have noticed that you feel hotter on a sunny afternoon as compared to a windy night. This is because of the difference in temperatures. Temperature is very high in the afternoon as compared to night. This chapter basically gives us the information about thermal properties of matter where we will study about the properties of different substances by virtue of heat/heat transfer.
Temperature and heat
Temperature is the relative measure or indication of the hotness and coldness of a body. A hot cooker is said to have higher temperatures and ice cubes to have lower temperature. An object at a higher temperature is said to be hotter than the one at a lower temperature. The S.I unit of Temperature is Kelvin (K).
A cup of hot soup and cold ice cream.
Heat
When we put a cold spoon into a cup of hot tea, the spoon warms up and the tea cools down as they were trying to equalize the temperature. Energy transfer that takes place solely because of temperature difference is called heat flow of heat transfer and the energy transferred is called heat. The S.I. unit of heat transfer is expressed in Joule (J).
Measurement of Temperature
A physical property that changes with temperature is called thermometric property. When a thermometer is put in contact with a hot body, the mercury expands, increasing the length of the mercury column, which can be calibrated and later be used to measure temperature.
This was one such example, there are many such which enable us to measure temperature.
There are three scales of measurement of Temperature.
- Celsius scale
- Fahrenheit scale
- Kelvin scale
The standard scale of measurement of temperature is Kelvin scale.
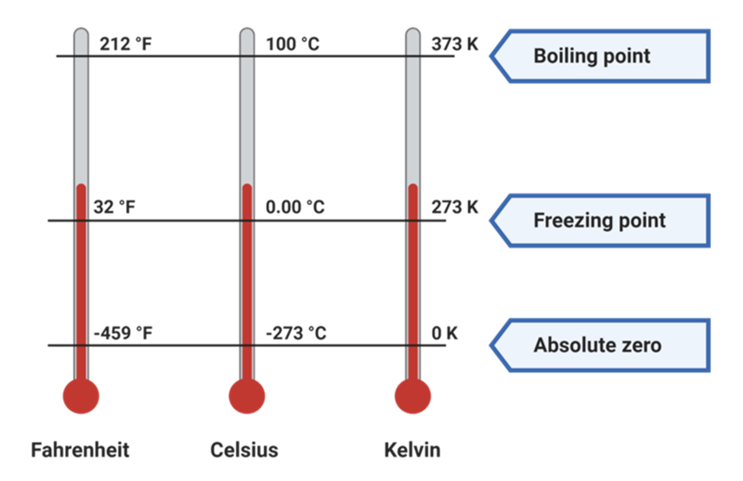
- Celsius scale: It defines the ice point at 0 degree Celsius and the steam point temperature as 100 degree Celsius. The space between 0 and 100 degree Celsius is equally divided into 100 intervals.
- Fahrenheit Scale: It defines the ice-point temperature as 32 F and the steam point is 212 F. The space between 32 F and 212 F is divided into 180 intervals.
- Kelvin scale: Kelvin scale is a scale of measuring temperature, the melting point of ice is taken as 273 K and the boiling point of water at 373 K. The space between these is divided in 100 intervals. This is also known as the absolute scale of temperature as it has only positive values of Temperature. This scale has been adopted as the standard scale of measuring Temperature.
To convert a temperature from one scale to the other, we must take into account the fact that the zero temperatures of the two scales are not the same. Below is the relation between different scales of temperature.
![]()
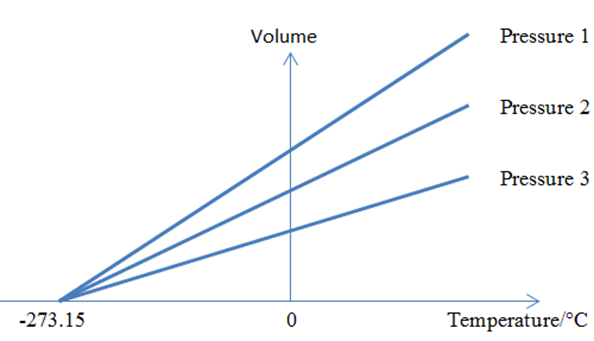
Ideal gas equations and absolute Temperature
We have Liquid-in-glass thermometers like mercury thermometers, these thermometers do not give accurate readings for temperature other than the ice point and boiling point because of differing expansion properties of liquid.
A thermometer that uses a gas however gives the same readings regardless of which gas is used. This is considered to be a more accurate thermometer than liquid-in-glass thermometer. Experiments show that all gases at low densities exhibit the same expansion behavior. The variables that describe the behavior of a gas of given quantity (mass) are
- Pressure
- Temperature
- Volume
There are some laws that are followed by gases of low density. These laws are:-
- Charles’s law: This law states that at constant pressure, volume and temperature of the gas are directly proportional for a fixed quantity of gases.
- Boyle’s law: This law states that at a constant temperature, the volume of the gas is inversely proportional to the pressure of gas for a fixed quantity of gases.
- Avogadro law: At constant Pressure and Temperature, equal volume of gases contains equal number of molecules of gas. In other words, we can say that at constant P and T, the Volume of the gas is directly proportional to the number of molecules of gas.
If we combine these three laws we will get
To remove the proportionality sign we add a constant R.
Above equation is called the ideal gas equation and R= constant of proportionality is called the gas constant.
Absolute zero temperature
This point, where all the atoms have been completely stopped relative to each other, is known as "absolute zero" and corresponds to the number zero on the Kelvin temperature scale. An object cannot be cooled below this point because there is no atomic thermal motion left to stop.
Can absolute zero ever be reached?
Physicists acknowledge they can never reach the coldest conceivable temperature, known as absolute zero.
The zero on a Kelvin scale is called the absolute zero. Absolute Temperature is equal to minus 273-degree Celsius or 459.67 degrees Fahrenheit.
Thermal expansion
Thermal expansion is the tendency of matter to change in shape, volume, and area in response to a change in temperature. Temperature is a monotonic function of the average molecular kinetic energy of a substance.
Thermal expansion is caused by heating solids, liquids or gases, which makes the particles move faster or vibrate more (for solids). This means that the particles take up more space and so the substance expands
The amount by which it expands depends on three factors: its original length, the temperature change, and the thermal (heat) properties of the metal itself. Some substances simply expand more easily than others.
Thermal expansion is of three types:
- Linear expansion. The expansion in length is called linear expansion.
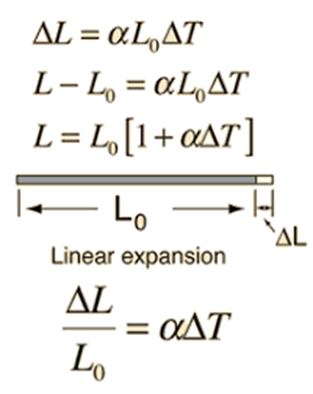
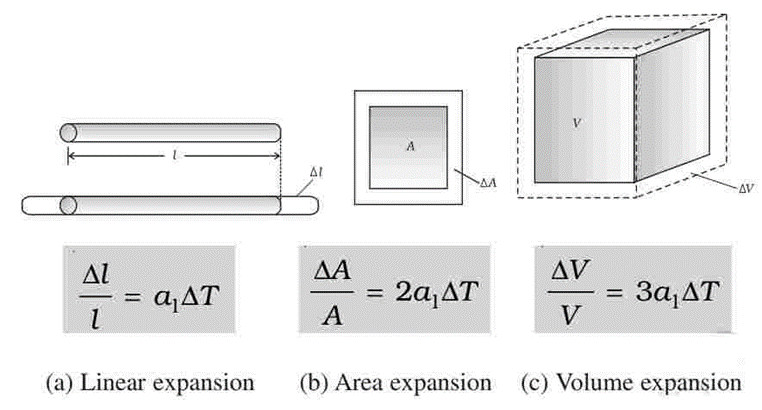
- Area expansion. The expansion in area is called area expansion.
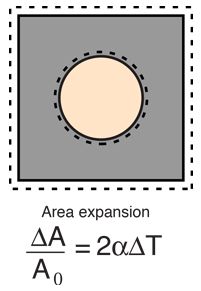
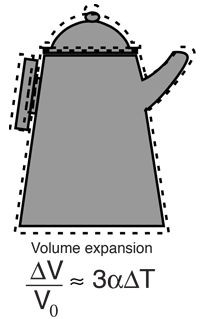
- Volume expansion. The expansion in volume is called volume expansion.
If the coefficient of linear expansion is denoted by α
Coefficient of area expansion is denoted by β
Coefficient of volume expansion is denoted by γ
The relation between α, β and γ is stated as
Anomalous behavior of water
Water shows some exceptional behavior that is when it is heated at 0°C, it contracts instead of expanding and it happens till it reaches 4 °C. The volume of a given amount of water is minimum at 4 °C therefore its density is maximum (Refer the Fig). After 4 °C water starts expanding. Below 4 °C, the volume increases, and therefore the density decreases. This means water has a maximum density at 4 °C.
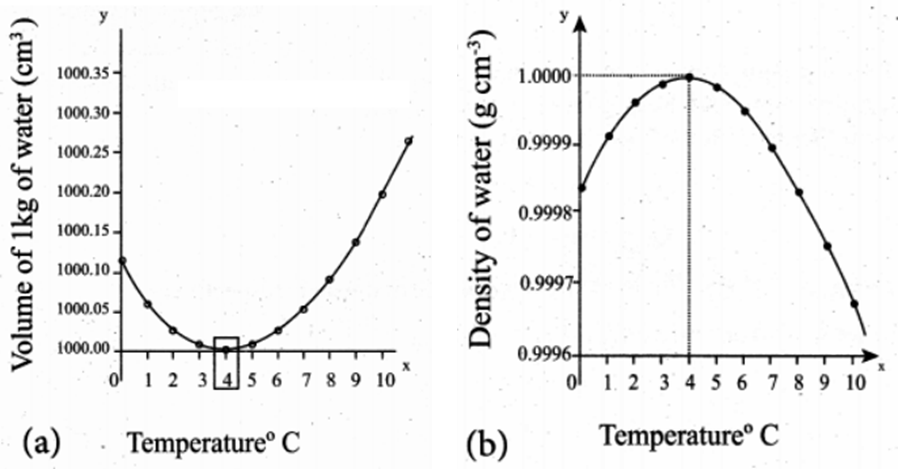
Density of water is maximum and the volume of water is minimum at 4 degree Celsius. This is anomalous behavior of water. Because of this property of water in lakes and ponds freeze only at the top layer and at the bottom it does not, but if the water freezes at the bottom also then animal and plant life would not be possible.
The anomalous behavior of water, sometimes called the density anomaly, is due to strong intermolecular attractions between water molecules called hydrogen bonds. The large electronegativity difference between oxygen and hydrogen causes the hydrogen-oxygen bonds to be polar.
Specific heat capacity
Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific heat are usually calories or joules per gram per Celsius degree. For example, the specific heat of water is 1 calorie (or 4.186 joules) per gram per Celsius degree.
But let's try to understand the specific heat in detail.
Suppose you have 100 g of water in a vessel at 20 C temperature and you put that vessel on top of a stove (source of heat). Place the thermometer inside it and hold a stopwatch. The heat from the stove will heat up the water and will raise its temperature that can be seen on the thermometer.
- First note the time for increasing the temperature of the water from 20 degrees Celsius to 40 degrees Celsius (rise of 20 C).
- Now you have water at 40 C, now again note the time for increasing the temperature of water upto 60 C (a rise of 40 C). You will notice that it takes double the time if we double the rise of temperature.
From the above experiment, we have the following conclusion.
Heat required to raise the temperature of a substance is proportional to the rise in temperature
Now we will do this experiment again but now with double quality. We now have 200 g of water at 20 C in a vessel which is kept on a stove and keeping all other things the same.
- If we now try to raise the temperature of it upto 40 C and note the time of it. We will notice that you need double the time as we got when we had only 100 g of water. (case 1)
Thus, Heat required to raise the temperature of the substance is proportional to the amount of substance.
Now in the next experiment, we change the liquid from water to oil. We take 100 g of oil at 20 C in a vessel keeping all other things the same.
- If we now try to raise its temperature upto 40 C and note the time of it. You will get the time that is very less as compared to we get in case 1
Thus, the Heat required to raise the temperature of the substance depends on the nature of the substance.
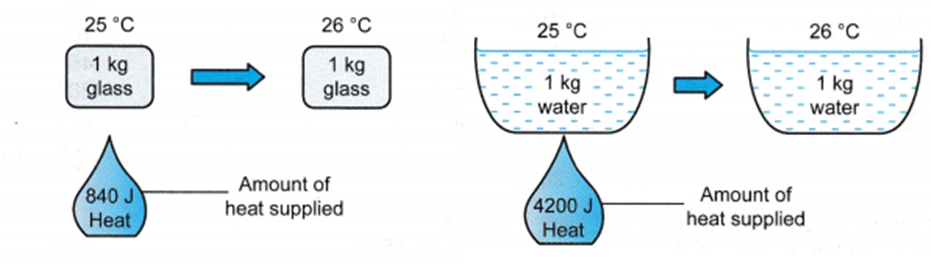
The above series of experiments shows that the quantity of heat required to warm a given substance depends on its mass, m, the change in temperature, ΔT and the nature of the substance. The change in temperature of a substance, when a given quantity of heat is absorbed or rejected by it, is characterized by a quantity called the heat capacity of that substance. Every substance has a fixed value of heat capacity.
Now you will understand the definition of specific heat more clearly.
Specific heat is defined as the amount of heat per unit mass absorbed or rejected by the substance to change its temperature by one.
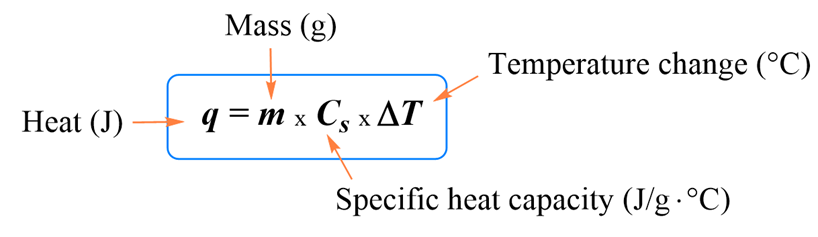
Molar heat capacity
Heat capacity per mole of the substance is defined as the amount of heat (in moles) absorbed or rejected (instead of mass m in kg) by the substance to change its temperature by one unit.
Here q= heat absorbed in Joules (J), n= number of moles,
Cm = molar specific heat expressed in
Heat transfer can be achieved by keeping either pressure or volume constant, accordingly, we define Cv and Cp. Let us discuss this now.
Molar-specific heat capacity at constant volume (Cv)
If the volume of the gas is maintained during the heat transfer, then the corresponding molar-specific heat capacity is called molar-specific heat capacity at constant volume (Cv).
Water has the highest specific heat of capacity because of which it is used as a coolant in automobile radiators and in hot water bags.
Molar-specific heat capacity at constant pressure (Cp)
If the gas is held under constant pressure during the heat transfer, then the corresponding molar-specific heat capacity is called molar-specific heat capacity at constant pressure (Cp).
A fun thing to do: Virtual lab
Below is the link of the states of matter simulations.
What can we do in this simulation?
- We can choose from the options of gases available ( Neon, Argon, Oxygen and water)
- Then we can choose the state of matter from solid, liquid and gas.
We can then observe the temperature at which these gases are in different states. Like if we choose Water and Gas then the temperature would be around 373K or more, which says that water will be in gaseous form at 373 K and above.
- We can also choose one gas and then raises or lower its temperature and with the help of the motion of its atoms or molecules we can say whether it is in liquid , gaseous or solid states

 Madhava Publications
Madhava Publications
