Introduction
Thermodynamics is that branch of physics which deals with concepts of heat and temperature and their relation to energy and work.
We can also consider it as a macroscopic science which deals with bulk systems and tells us about the system as a whole.
The foundation of thermodynamics is the conservation of energy and the fact that the heat flows spontaneously from hot to the cold body and not the other way around. The study of heat and its transformation to mechanical energy is called thermodynamics. It comes from a Greek word meaning “Movement of heat”.
A collection of large numbers of molecules of matter (solid, liquid or gas) that are arranged in a manner such that these possess particular values of pressure, volume and temperature form a thermodynamic system.
The distinction between mechanics and thermodynamics is worth bearing in mind. In mechanics, our interest is in the motion of particles or bodies under the action of forces and torques. Thermodynamics is not concerned with the motion of the system as a whole. It is concerned with the internal macroscopic state of the body.
In this chapter, we will learn about the laws of thermodynamics which describes the system in terms of macroscopic variables, and reversible and irreversible processes. Finally, we will also learn on what principle heat engines, refrigerators and Carnot engines work.
Thermal equilibrium
Equilibrium in mechanics means that the net external force and torque on a system are zero. The term ‘equilibrium’ in thermodynamics appears in a different context: we say the state of a system is an equilibrium state if the macroscopic variables that characterize the system do not change in time.
For example, a gas inside a closed rigid container, completely insulated from its surroundings, with fixed values of pressure, volume, temperature, mass and composition that do not change with time, is in a state of thermodynamic equilibrium
Consider two bodies at different temperatures one is at 30 C and another at 60 C then the heat will flow from the body at a higher temperature to the body at a lower temperature. Heat will flow till both bodies acquire the same temperature. This state when there is no heat flow between two bodies when they acquire the same temperature is known as thermal equilibrium.
Types of Equilibrium
Thermal Equilibrium: - Two systems are said to be in thermal equilibrium with each other if the temperatures of both systems do not change with time.
Chemical Equilibrium: - Two systems are said to be in chemical equilibrium with each other if the composition of the system does not change over time.
Mechanical Equilibrium: - Two systems are said to be in mechanical equilibrium with each other if the pressure of the system doesn’t change with time.
A system is said to be in Thermodynamic equilibrium when all of its macroscopic variables are constant.
System and surrounding
System: - System is defined as any part of the universe enclosed by some boundary through which exchange of heat or energy takes place.
Surroundings: - Any part of the universe which is not a system. Systems and surroundings constitute the Universe.
For example: -
- If we consider hot coffee in a kettle then the kettle is the system and everything else is the surroundings.

- A cup of hot coffee after some time becomes cold due to the exchange of heat between the system and surroundings.
Types of system
A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. There are three types of systems:
- Isolated System – An isolated system cannot exchange energy and mass with its surroundings. The universe is considered an isolated system. For example a thermos flask
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place.
For example A balloon filled with gas, A pot with a lid etc.

- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings.
For Example: - Water boils in a pan without lid, a cup of coffee etc.
Types of walls
Adiabatic wall: - It is an insulating wall that doesn’t allow heat to flow from one system to another. This means the temperature of both the systems won’t change with time.
Consider 2 systems A and B as shown in the figure, which are separated by adiabatic walls. Let the pressure and volume of A be (P1, V1) and (P2, V2).
Both these systems are also separated from the surroundings by an adiabatic wall which means there is no flow of heat between A and surroundings and also B and surroundings.
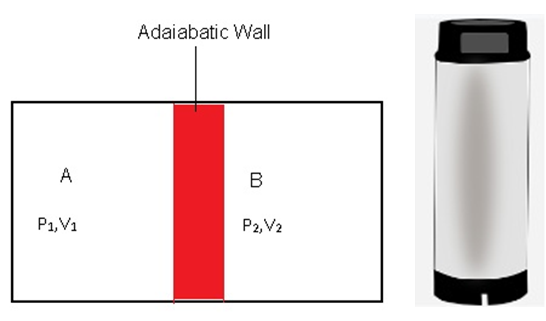
For example: - Thermos Flask. In which tea or coffee remains hot for a long time as it is made of insulating walls due to which there is no heat flow between tea and surroundings.
Diathermic (conducting) Wall: - It is a conducting wall that allows the flow of heat between any 2 systems.
- Consider two systems A and B which are separated by a conducting wall. System A is at higher temperature T1, pressure P1 and volume V1 and System B is at lower temperature T2, pressure P2 and volume V2.
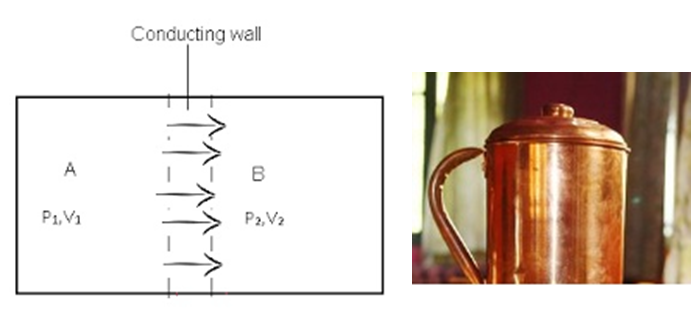
- There is flow of heat from a system at a higher temperature to the system at a lower temperature till the systems reach thermal equilibrium.
For Example: - A vessel made up of metals like copper or aluminum has diathermic / conducting walls
Zeroth law of thermodynamics: Temperature
Zeroth law of thermodynamics states that when two systems are in thermal equilibrium through a third system separately then they are in thermal equilibrium with each other also.
Forge: - Consider two systems A and B which are separated by an adiabatic wall. Heat flow happens between systems A and C, and between B and C, due to which all 3 systems attain thermal equilibrium.
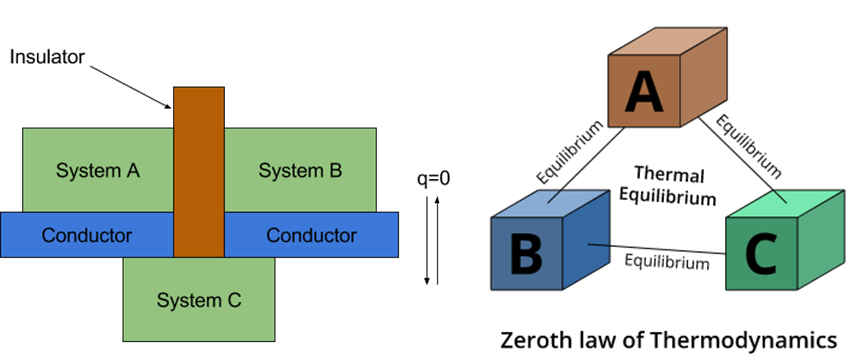
Systems A and B are in thermal equilibrium with C. Then they will be in equilibrium with each other also.
- Zeroth's Law of Thermodynamics suggested that there should be some physical quantity that should have the same value for the system to be in thermal equilibrium.
- This physical quantity that determines whether a system is in equilibrium or not is Temperature.
- Temperature is the quantity that determines whether the system is in thermal equilibrium with the neighboring system.
- When the temperature becomes equal then the flow of heat stops.
Thermodynamic state variable
Thermodynamic state variables are the macroscopic quantities which determine the thermodynamic equilibrium state of a system. These macroscopic quantities are known as thermodynamics state variables.
- As they determine the state of the system, that is pressure, volume and temperature, at one particular time they are known as thermodynamic state variables. Pressures (P), Volume (V), Temperature (T), mass (m), and Internal energy (U) are the thermodynamic state variables.
- These variables can tell us the position or the condition of any gas at that particular time.
- A system not in equilibrium cannot be described by state variables. It means the macroscopic variables are changing with time and they are not constant.
Types of thermodynamic state variables:-
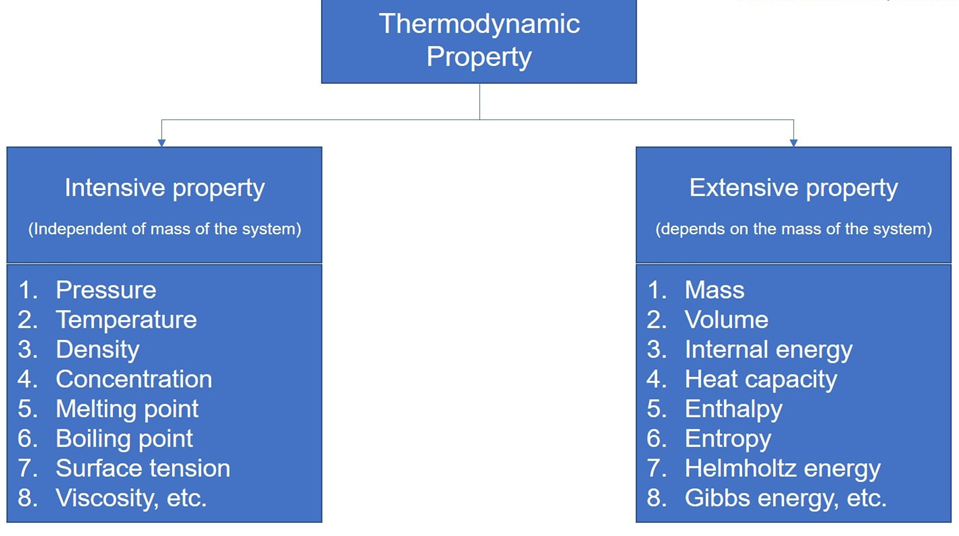
- Extensive variables: - They indicate the size of the system, which means extensive variables are those that depend on the mass of the system or the number of particles in the system. Example: volume, mass, internal energy. If we consider a system whose mass is greater than the size of that system is greater. All these depend on the size of the system.
- Intensive variables: -A quantity in a macroscopic system that has a well-defined value at every point inside the system and that remains (nearly) constant when the size of the system is increased. Examples of intensive variables are pressure, temperature, density, specific heat capacity at constant volume, and viscosity.
In the figure given below various examples of extensive and intensive variables are given.
Internal Energy
It is defined as the sum of kinetic energies and potential energies of the molecules constituting the system as a whole and not of individual molecules. It is a macroscopic variable of the system.
It is denoted by U. It is an extensive thermodynamic state variable as it depends on the size of the system.
It only depends on the state of the system at that particular time and does not depend on how the system has reached that state.
There are two modes of changing the internal energy of a system
- Heat
- Work
Consider again, for simplicity, the system to be a certain mass of gas contained in a cylinder with a movable piston. There are two ways to change the internal energy of the system.
By Heat: One way is to put the cylinder in contact with a body at a higher temperature than that of the gas. The temperature difference will cause a flow of energy (heat) from the hotter body to the gas, thus increasing the internal energy of the gas.
By Work: The other way is to push the piston down i.e. to do work on the system, which again results in increasing the internal energy of the gas.
Both these things could happen in the reverse direction. With surroundings at a lower temperature, heat would flow from the gas to the surroundings. Likewise, the gas could push the piston up and do work on the surroundings.
Few things to be remembered:
The notion of heat should be carefully distinguished from the notion of internal energy. Heat is certainly energy, but it is the energy in transit. The state of a thermodynamic system is characterized by its internal energy, not heat.
A statement like ‘a gas in a given state has a certain amount of heat’ is as meaningless as the statement that ‘a gas in a given state has a certain amount of work’. In contrast, a gas in a given state has a certain amount of internal energy is a perfectly meaningful statement. Similarly, the statements ‘a certain amount of heat is supplied to the system’ or ‘a certain amount of work was done by the system’ are perfectly meaningful.
First Law of thermodynamics
According to the first law of thermodynamics: - The change in the internal energy of a closed system is equal to the amount of heat supplied to the system, minus the amount of work done by the system on its surroundings.
Mathematically,
Sign conventions:
- When the heat gets supplied to the system, then ΔQ is taken positive and when heat gets withdrawn from the system, ΔQ is taken negative.
- When a gas expands, work done by the gas is taken positive whereas when a gas contracts, work is taken negatively. Work done is also a path variable so its value also depends on the path chosen.
- ΔU is taken positively when temperature increases while ΔU is taken negative when temperature decreases.
Remember, Heat ΔU and work done is a path variable so it depends on the path chosen. Internal energy is a state variable so its value doesn’t depend on the path followed but only depends on the initial and final state of the system.
Limitations of the first law of thermodynamics
The first law of thermodynamics plays an important role in thermodynamics as it can be applied to know how much work will be obtained by transferring a certain amount of heat energy in a given thermodynamics process. However, the first law of thermodynamics suffers from the following limitations.
- First law of thermodynamics does not indicate the direction of heat transfer.
- First law of thermodynamics does not tell anything about the conditions under which heat can be transformed into work.
- The first law does not indicate why the whole of the heat energy cannot be continuously converted into mechanical work.

 Madhava Publications
Madhava Publications
