1. Group 13 elements : The boron family
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER- 11 THE p BLOCK ELEMENTS
• p-Block Elements
Elements belonging to groups 13 to 18 of the periodic table are called p-block elements. General electronic configuration: ns2 np1-6 (except for He)
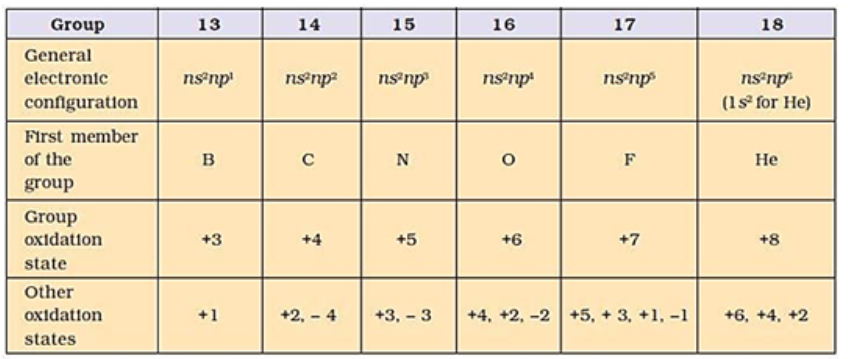
GROUP 13 ELEMENTS: THE BORON FAMILY
Outer Electronic Configuration: ns2np1
Atomic Radii: The atomic and ionic radii of group 13 elements are smaller than the corresponding elements of alkali and alkaline earth metals.
Reason: On moving from left to right in a period the effective nuclear charge increases and the outer electrons are pulled more strongly towards the nucleus. This results in decrease in atomic size.
On moving down the group, both atomic and ionic radii expected to increase due to the addition of a new electron shell with each succeeding element.
Exception: Atomic radius of Ga is less than that of Al due to the presence of poor shedding 10d-electrons in gallium.
Ionisation enthalpies: First ionisation enthalpies of the elements of group-13 are less than those of the elements present in group-2 in the same period.
Reason: The removal of p-electron is much easier than the s-electron and therefore, the first ionisation enthalpies (∆i H1) of the elements of group 13 are lower as compared to the corresponding elements of group 2.
On moving down the group 13 from B to Al the first-ionization enthalpies (∆i H1) decrease due to an increase in atomic size and screening effect which outweigh the effect of increased
nuclear charge.
There is discontinuity expected in the ionisation enthalpy values between Al and Ga and between In and Tl due to inability of d- and f-electrons which have low screening effect to compensate the increase in nuclear charge.
Electronegativity: Down the group, electronegativity first decreases from B to Al and then increases.
This is due to discrepancies in the atomic size of the elements.
Physical Properties
(i) Due to strong crystalline lattice boron has high melting point. Rest of the members of this family have low melting point.
(ii) Boron is extremely hard and black coloured solid and non metallic in nature.
(iii) Other members of this family are soft metals with low melting point and high electrical conductivity.
Chemical Properties
Oxidation states: The first two elements boron and aluminium show only +3 oxidation state ~ in the compounds but the other elements of this group gallium, indium and thalium also exhibit +1 oxidation state in addition to +3 oxidation state i.e., they show variable oxidation states.
As we move down the group, the stability of +3 oxidation state decreases while that of +1 oxidation state progressively increases.
2. Important trends and anomalous properties of boron
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
IMPORTANT TRENDS AND ANOMALOUS PROPERTIES OF BORON
Trichloride, Tribromides and Triiodides of group 13 elements are covalent in nature and can be hydrolysed in water.
Monomeric trihalides of these elements are strong Lewis acids.
BF3 + :NH3 → NH3-BF3
Metal halides of group 13 elements are generally dimerized through halogen bonding.
Anomalous properties of Boron are listed below –
Boron shows quite higher melting and boiling points than other elements of group 13.
Boron forms only covalent compounds while other elements of the group 13 form both ionic and covalent compounds.
Boron is a metalloid while other elements of the group 13 are metals.
Oxides and hydroxides of boron are acidic in nature while oxides and hydroxides of other elements of the group are amphoteric and basic in nature.
3. Some important compounds of boron
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME COMPOUNDS OF BORON
Structure of boric acid
It has a layer structure in which planar BO3 units are joined by hydrogen bonds as shown in Fig.
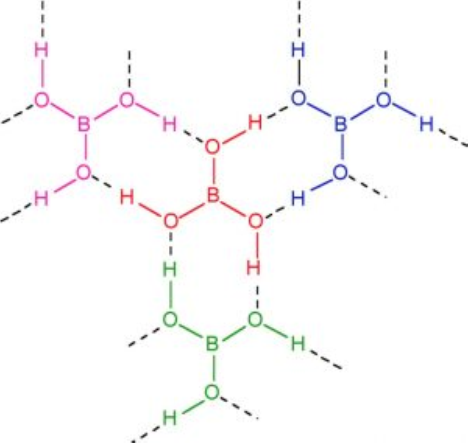
Physical properties of boric acid:
(i) It is a white crystalline solid.
(ii) It is soft soapy in touch.
(iii) It is sparingly soluble in cold water but fairly soluble in hot water.
Uses:
(i) In the manufacture of heat resistant borosilicate glazes.
(ii) As a preservative for milk and food stuffs.
(iii) In the manufacture of enamels and glazes in pottery.
(iii) Diborane, (B2H6): The series of compounds of boron with hydrogen is known as boranes.
Diborane is prepared by the reduction of boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 ——> 2B2H6+ 3LiF + 3AlF3
Laboratory method of preparation. In laboratory diborane is prepared by the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 ——> B2H6 + 2NaI +H2
Industrial method of preparation. On industrial scale, diborane is prepared by reduction of BF3 with sodium hydride.
![]()
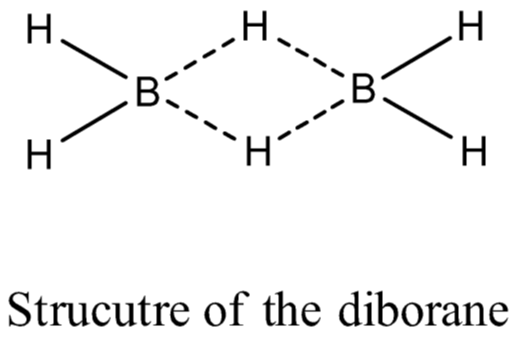
Physical Properties:
(i) Diborane is a colourless, highly toxic gas with a b.p. of 180 K.
(ii) Diborane catches fire spontaneously upon exposure to air.
(iii) Higher boranes are spontaneously flammable in air.
Chemical properties:
(i) Boranes are readily hydrolysed by water to form boric acid
B2H6(g) + 6H20(Z) ——> 2B(OH)3(aq) + 6H2(g)
(ii) It burns in oxygen evolving an enormous amount of heat
B2H6 + 302 —–> B203 + 3H20
(iii) Reaction with Lewis base:
Diborane on treatment with lewis bases undergo cleavage reactions to form borane which then reacts with Lewis bases to form adducts.
B2H6 + 2NMe3 ——> 2BH3. NMe3
B2H6 + 2CO ———> 2BH3 .CO
4. Uses of boron and aluminium and their compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
USES OF BORON AND ALUMINIUM AND THEIR COMPOUNDS
Uses of Boron
1) Boron fibres have enormous tensile strength and hence are used to make bullet proof vests and light composite material for aircraft.
2) Natural boron is 20% boron-10 and 80% boron-11.Boron-10 has a high cross section for absorption of low energy neutrons.
3) Borax and boric acid are used in the manufacture of heat resistant glass, glass wool and fibre glass. Borax is also used as a flux in soldering ,as a constituent of medicinal soaps due to its antiseptic properties ,in the manufacture of enamels and glazes for earthenware’s i.e. tiles, pottery etc. An aqueous solution of orthoboric acid is also used as a mild antiseptic.
4) It is used in steel industry for increasing the hardness of Steel. In fact boron has replaced expensive metals like Mo, Cr and W in with manufacture of special hard steels.
5) Boron compounds are being used as rocket fuel because of high energy/ mass ratio.
Uses of aluminium
Aluminium is a bright silvery white metal with high tensile strength.
1) It is used for making transmission cables and for winding the moving coils of dynamos or motors.
2) It is used for making aluminium paint for protection of iron and zinc. Aluminium powder mixed with linseed oil shines like silver and hence is called silver paint.
3) Aluminium is a cheap metal which resist corrosion. Therefore ,it is used for making household utensils ,cans for drinks, tubes for toothpaste, pictures frames, trays etc. It is also used in building for making angles for doors, windows.
4) Aluminium foil is used for wrapping fine articles like photographic films , pharmaceutical products ,cigarettes sweets.
5) Aluminium powder is used as a reducing agent in aluminothermic process for extraction of chromium and manganese from their ores.
6) Aluminium powder is used in flash light bulbs for indoor photography.
7) Large amount of aluminium are converted into alloys. Some important alloys of aluminium are Aluminium bronze (used in coins, utensils, jewellery, picture frame), Magnalium (used in light instruments, balance beams, pressure cookers) , duralumin (used in automobile parts, pressure cooker)
8) Al(OH)3 is widely used as an antacid for treatment of digestion.
9) Anhydrous AlCl3 is used as a catalyst in Friedel – Crafts reaction and in cracking of petroleum. Hydrated AlCl3 is used as a mordant in dyeing.
10) Potash alum is used for purification of water, as styptic for stopping bleeding , in form type fire extinguisher, as mordant for dyeing and for tanning of leather and sizing of paper.
5. Group 14 elements : The carbon family
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GROUP 14 ELEMENTS: THE CARBON FAMILY
Group 14 includes carbon (C), silicon (Si), Germanium (Ge), tin (Sn) and lead (Pb).
General electronic configuration of carbon family is ns2np1.
Carbon: Carbon is the seventeenth most abundant element by weight in the earth’s crust.
(i) It is available as coal, graphite and diamond. In combined state it is present in metal carbonates, hydrocarbons and carbon dioxide gas (0.03%) in air.
(ii) Naturally occurring carbon contains two stable isotopes 12C and 13C and third isotope 14C. 14C is a radioactive isotope with half life 5770 years and is used for radiocarbon dating.
Covalent radius: Covalent radius expected to increase from C to Si. From Si to Pb small increase is found.
Reason: Due to the addition of a new energy shell in each succeeding element. The increase in covalent radii from Si to Pb is small due to ineffective shielding of the valence electrons by the intervening d- and f orbitals.
Ionization Enthalpy: The first ionization enthalpies of group 14 elements are higher than those of the corresponding group 13 elements.
Reason: Because effective nuclear charge increases and size of the atoms becomes smaller. First ionization enthalpy decreases on moving down the group from carbon to tin.
The decrease is very sharp from carbon to silicon while there is slight increase in the first ionization enthalpy of lead as compared to that of tin.
Electronegativity: Group 14 elements are smaller in size as compared to group 13 elements that’s why this group are slightly more electronegative than group 13. From Si to Pb it is almost same. Small increase in ionization enthalpy from Sn to Pb is due to the effect of increased nuclear charge outweighs the shielding effect due to the presence of additional 4f- and 5d-electrons.
Physical properties:
(i) All the elements of group 14 elements are solids. They are less metallic than group 13.
(ii) M.P. and boiling points of group 14 elements are generally high.
Chemical properties:
Carbon and silicon mostly show +4 oxidation state. Germanium forms stable compounds in +4 state and only few compounds in +2 state.
Tin forms compounds in both oxidation states. Lead forms compounds in +2 state are stable and in +4 state are strong oxidising agents.
6. Important trends and anomalous properties of carbon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
IMPORTANT TRENDS AND ANOMALOUS BEHAVIOUR OF CARBON
Carbon, differs from the rest of the member of its family. The main reason for the anomalous behaviour is:
(i) exceptionally small atomic and ionic size
(ii) higher ionization enthalpy
(iii) absence of d-orbitals in the valence shell.
(iv) Higher electronegativity.
It can be explained as follows:
=> Since carbon has only s and p-orbitals it can accommodate only four pairs of electrons ; other member can expand their covalence due to the presence of d-orbitals.
=> Carbon can form Pπ-Pπ multiple bonds with itself and other atoms having small size and high electronegativity.
For example, C=C, C≡C, C=O, C=S and C≡N
The order of catenation is C >> Si > Ge ≈ Sn
Heavier elements do not form Pπ-Pπ bonds because their atomic orbitals are too
large and diffuse to have effective overlapping.
=> Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings. This property is called catenation.
Down the group property to show catenation decreases.
C > Si > Ge > Sn > Pb
Lead does not show catenation.
7. Allotropes of carbon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALLOTROPES OF CARBON
The property of an element to exist in two or more forms which have different physical properties but identical chemical properties is called allotropy and different forms are called allotropes. Carbon exists in two allotropic forms:
(i) Crystalline
(ii) Amorphous
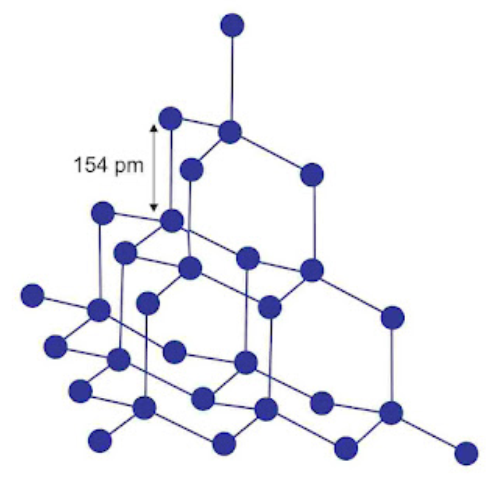
Crystalline form of carbon: Diamond, Graphite, Fullerenes Diamond: In diamond each carbon atom undergoes sp3 hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms. The C—C bond length is 154 pm.
Properties:
(i) It is the hardest substance on earth.
(ii) It is used as an abrasive for sharpening hard tools in making dyes and in manufacture of tungsten filaments.
Graphite: In graphite, carbon is sp2-hybridized. Graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Layers are held by van der Waals forces and distance between two layers is 340 pm.
Properties:
(i) Graphite conducts electricity along the sheet.
(ii) It is very soft and slippery.
(iii) Used as a dry lubricant in machines running at high temperature, where oil cannot be used as a lubricant.
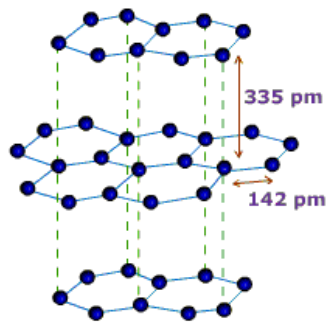
Fullerenes: Fullerenes was discovered collectively by three scientists namely E. Smalley, R.F. Curl and H.W. Kroto.
Preparation:
Fullerenes is prepared by heating of graphite in an electric arc in the presence of inert gas such as helium or argon.
The sooty material formed by the condensation of vapourised Cn small molecules consists of mainly with smaller quantity of C70 and traces of other fullerenes consisting of even number of carbon atoms up to 350or above.
Fullerenes are cage like molecules. C60 molecule has a shape like soccer ball and called Buckminsterfullerene’s. It is the most stable.
It contains 20 six-membered rings and 12 five-membered rings.
Six-membered rings are fused to both the other six-membered rings and five-membered rings but the five-membered rings are connected only to six-membered rings.
All the carbon atoms are equal and they undergo sp2-Kybridization.
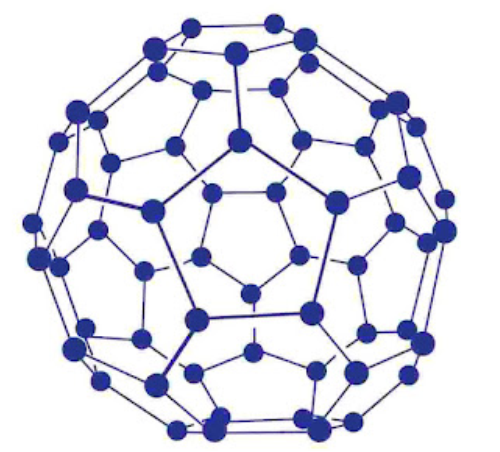
Properties:
(i) Fullerenes being covalent are soluble in organic solvents.
(ii) It also forms platinum complexes.
Amorphous allotropic forms of carbon coke: It is a greyish black hard solid and is obtained by destructive distillation.
Wood charcoal: It is obtained by strong heating of wood in a limited supply of air.
Animal charcoal: It is obtained by the destructive distillation of bones.
Uses of carbon:
(i) Graphite fibre are used for making superior sports goods such as tennis and badminton rackets, fishing rods.
(ii) Being good conductor graphite is used for making electrodes for batteries and industrial electrolysis.
(iii) Being highly porous, activated charcoal is used for absorbing poisonous gases in gas masks. It is used to decolourize sugar.
(iv) Carbon black is used as black pigment in black ink and as filler in automobile tyres.
(v) Coke is extensively used as reducing agent in metallurgy.
(vi) Diamond is a precious stone.
8. Some important compounds of carbon and silicon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF CARBON AND SILICON
Carbon Monoxide
Preparation: It is prepared by direct oxidation of C in limited supply of oxygen.
2C + O2 → 2CO
Properties:
(i) Carbon monoxide is a colourless and odourless gas.
(ii) It is almost insoluble in water.
(iii) It is powerful reducing agent and reduces almost all metal oxides except alkali and alkaline earth metal oxides.
(iv) In CO molecule there are one σ (sigma) and two π bonds between carbon and oxygen.
: C = O :
(v) It is highly porous in nature. It forms a complex with haemoglobin which is about 300 times more stable than the oxygen-haemoglobin complex. This prevents haemoglobin in the red blood corpuscles from carrying oxygen round the body, thereby causing suffocation ultimately leading to death.
Carbon Dioxide
Preparation: It is prepared by complete combustion of carbon and carbon containing fuels in
- CaCO3 + 2HCl → CaCl2 + H2O + CO2
- CaCO3 → CaO + CO2
Structure:
Carbon dioxide, or CO2, has three resonance structures, out of which one is a major contributor.
The CO2 molecule has a total of 16 valence electrons - 4 from carbon and 6 from each oxygen atom.
Here are the three resonance structures for CO2, all accounting for the 16 valence electrons
The atoms in all three resonance structures have full octets; however, structure 1 will be more stable, and thus contribute more, because it has no separation of charge.
Structures 2 and 3 show charge separation caused by the presence of formal charges on both oxygen atoms. Moreover, the presence of a positive charge on oxygen further reduces the stability of these two structures.
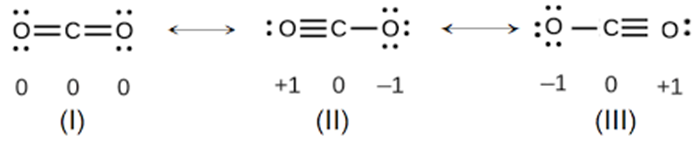
Properties:
(i) It is a colourless and odourless gas.
(ii) It is slightly soluble in water. When C02 dissolves in water only some of the molecules react with water to form carbonic acid.
(iii) It is not poisonous like CO.
But increase in combustion of fossil fuels and decomposition of limestone for cement manufacture increase of C02 in the atmosphere is one of the main reasons of green house effect.
Silicon dioxide (Si02)
Silicon dioxide, commonly known as silica, occurs in various crystallographic forms.
For example, Quartz, Cristobalite and thermite are some of the crystalline forms of silica.
Structure:
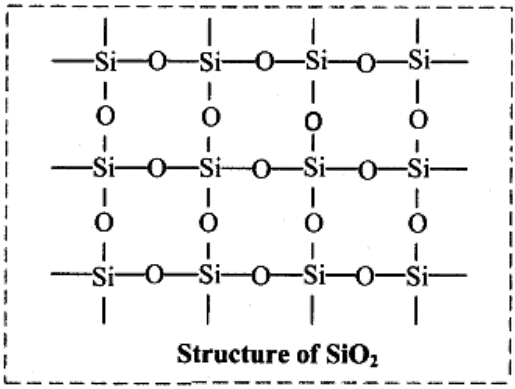
Silicon dioxide is a covalent three dimensional network solid.
Each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms.
Each oxygen atom in turn covalently bonded to another silicon atoms as shown below
Properties:
(i) In normal form silica is very less reactive.
(ii) At elevated temperature it does not reacts with halogens, dihydrogen and most of the acids and metals. But it reacts with HF and NaOH.
Si02 + 2NaOH —–> Na2Si03 + H2O
Si02+ 4HF ——–> SiF4+ 2H20
Uses:
(i) Quartz is extensively used as a piezoelectric material.
(ii) Silica gel is used as adsorbent in chromatography.
(iii) An amorphous form of silica, kieselguhr is used in filtration plants.
Zeolites
1. Zeolites are three dimensional crystalline solids containing aluminium, silicon and oxygen in their regular three-dimensional framework.
2. They are hydrated sodium alumino silicates with general formula, Na2O. (Al2O3). x(SiO2)y(H2O) (x = 2 to 10; y = 2 to 6)
3. Zeolites have porous structure in which the monovalent sodium ions and water molecules are loosely held.
4. The Si and Al atoms are tetrahedrally coordinated with each other through shared oxygen atoms.
5. Zeolites structure looks like a honeycomb consisting of a network of interconnected tunnels and cages.
6. Zeolite crystal to act as a molecular sieve. They helps to remove permanent hardness of water.
• P-Block elements: Contains, metals, non-metals and metalloids.
• General configuration: ns2np1-6
– Boron is a typical non-metal and the other members are metals.
– Boron halides are considered to behave like Lewis acids.
– Boric acid is a Lewis acid.
– Borax is a white crystalline solid formula is Na2 [B4O5(OH)4] . 8H20
– Aluminium exhibits +3 oxidation state.
– Allotropy: The important allotropes of carbon are diamond, graphite, and fullerenes.
– The members of carbon family exhibit +4 and +2 oxidation state. The tendency to show +2 oxidation state increases among heavier elements.
– Lead in +2 state is stable whereas in +4 oxidation state it is a strong oxidising agent.
– Carbon monoxide is neutral whereas C02 is acidic in nature.
– Carbon monoxide having lone pair of electrons on C forms metal carbonyls.
– Carbon monoxide forms a haemoglobin complex which is deadly poisonous due to its higher stability.
– Zeolites are complex aluminium silicates.

 Ritan Sheth
Ritan Sheth
