- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
QUALITATIVE ANALYSIS OF ORGANIC COMPOUNDS
An organic compound mainly contains carbon and hydrogen. Some compounds may also contain oxygen, nitrogen, sulphur, halogens and phosphorus.
Detection of Carbon and Hydrogen
Organic compound is heated with copper (II) oxide [CuO]. Carbon present in the compound is oxidised to carbon dioxide and hydrogen to water. CO2 can be tested by passing through lime-water, which turns milky and water can be tested with anhydrous copper sulphate, which turns blue.
C + 2CuO ⎯⎯Δ → 2Cu + CO2
2H + CuO ⎯⎯Δ → Cu + H2O
CO2 + Ca(OH)2 ⎯→ CaCO3↓ + H2O
Detection of Nitrogen, Sulphur and Halogens
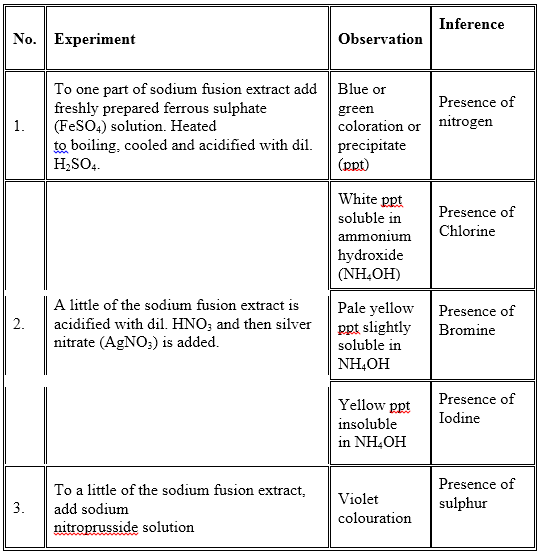
Nitrogen, sulphur and halogens present in an organic compound are detected by “Lassaigne’s test”. Here the organic compound is fused with metallic sodium in a fusion tube. It is then plunged into distilled water taken in a china dish. The solution is boiled and filtered. The filtrate is known as sodium fusion extract.
Principle: In an organic compound, nitrogen, sulphur and halogen atoms are present in covalent form. By heating with metallic sodium, these elements are converted to ionic form as follows:
Na + C + N ⎯⎯Δ → NaCN
2Na + S ⎯⎯Δ → Na2S
Na + X ⎯⎯Δ → Na X (X = Cl, Br or I)
For the detection of the elements, the following tests are done:
Test for Phosphorus
The organic compound is heated with an oxidising agent like sodium peroxide. The phosphorus present in the compound is oxidised to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.

 Ritan Sheth
Ritan Sheth
