- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
WATER
Human body has about 65% and some plants have nearly 95% water.
Physical properties of water:
(i) Freezing point of water is 273.15 K and boiling point 373.15 K.
(ii) Maximum density of water at 4°C is 1 gm cm-3
(iii) It is a colourless and tasteless liquid.
(iv) Due to hydrogen bonding with polar molecules, even covalent compounds like alcohol and carbohydrates dissolve in water.
Structure of Water:
In gas phase, it is a bent molecule with HOH bond angle 104.5° and O—H bond length of 95.7 pm. It is highly polar in nature. Its orbital overlap picture is also shown below.
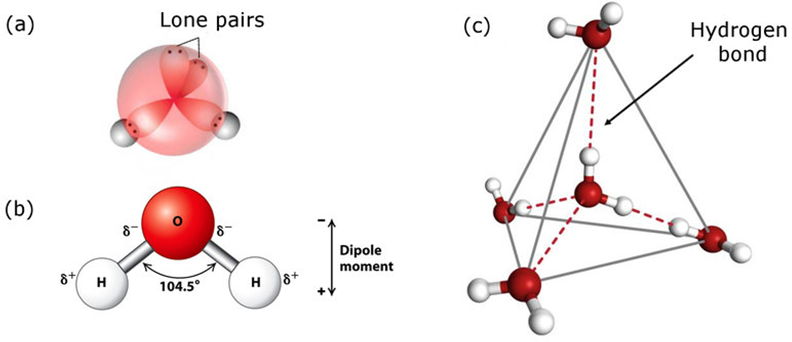
Water in Crystalline Form:
Ice is the crystalline form of water. At atmospheric pressure ice crystallise in the hexagonal form. At low temperature it condenses to cubic form. Density of ice is less than that of water. Therefore, ice cubes can float on water.
Structure of ice:
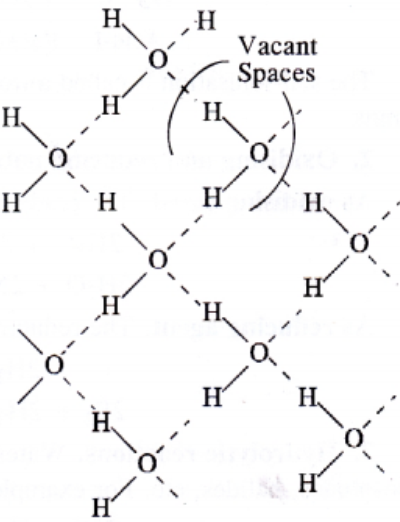
Chemical Properties of Water:
(i) Amphoteric nature: It behaves like an amphoteric substance because it can act as an acid as well as base.
H2O + HCl → H3O+ + Cl-
Autoprotolysis of water also accounts for its amphoteric nature according to Bronsted-Lowry concept.
H2O(l) + H2O(l) ↔ H3O+(aq) + OH-(aq)
pH of water is 7 and it is neutral towards pH.
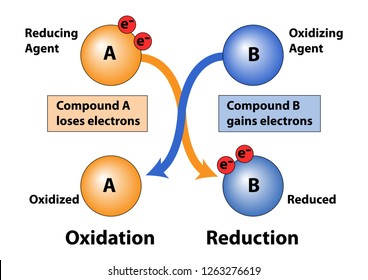
(ii) Oxidising and Reducing Nature: Water can act as an oxidising as well as reducing agent
(iii) Hydrolysis Reaction: It has a very strong hydrating tendency. It can hydrolyse a large number of compounds such as oxides, halides, carbides etc.

• Hydrates Formation
From aqueous solutions many salts can be crystallised as hydrated salts. Hydrates are of three types:
(i) Coordinated water
For example: [Ni(H20)6]2+ (N03–)2 and [Cr(H20)6]3+ 3CP
(ii) Interstitial water
For example: BaCl2. 2H20
(iii) Hydrogen bonded water
For example: [Cu(H20)4]2+ S042- H20 in CuS04.5H2
• Hard and Soft Water
Hard water: Water which does not produce lather with soap easily is called hard water. Presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride and sulphate in water makes the water hard.
Types of Hardness of Water:
(i) Temporary hardness: It is due to the presence of bicarbonates of calcium and magnesium in water. It is known as temporary because it can be easily removed by simple boiling of hard water.
(ii) Permanent hardness: It is due to the presence of chlorides and sulphates of calcium and magnesium. It cannot be removed on boiling water. Permanent hardness of water can be removed by chemical methods.
Soft water: Water which readily forms lather with soap is called soft water.
For example: rain water, distilled water.

 Ritan Sheth
Ritan Sheth
