- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ISOMERISM
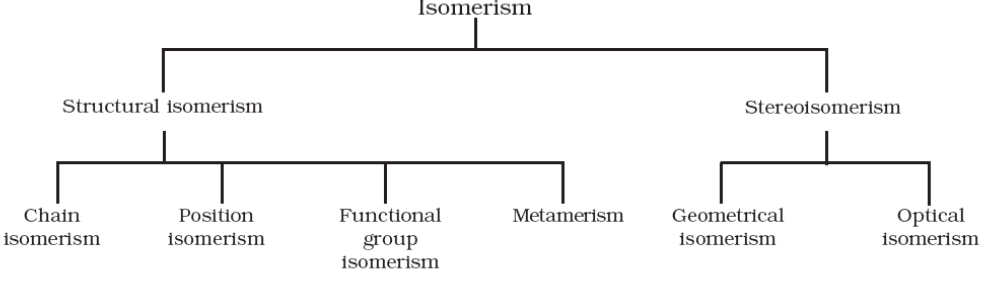
The phenomenon of existence of two or more compounds having the same molecular formula but different structural formula or spatial arrangement of atoms is known as isomerism. Such compounds are called as isomers. Isomers have different physical and chemical properties. Isomerism can be broadly classified into two – structural isomerism and stereo isomerism.
1. Structural isomerism
Compounds having same molecular formula but different structural formula (arrangement of atoms) are called structural isomers and the phenomenon is called structural isomerism. There are mainly four types of structural isomerism:
- Chain Isomerism: Isomers differ in carbon chain or skeleton are called chain isomers and the phenomenon is called chain isomerism.
E.g.: Pentane(C5H12)
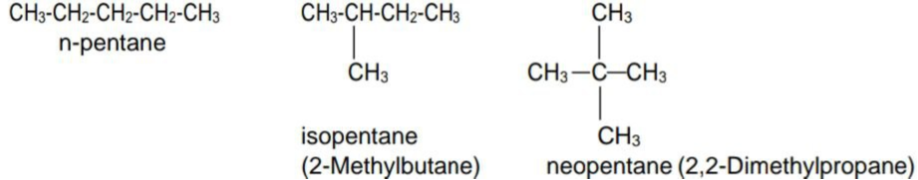
- Position isomerism: Isomers which differ in the position of the substituent or side chain are called position isomers and the phenomenon is called position isomerism.
E.g. : Alcohol with molecular formula C4H10O may be 1-butanol or 2 butanol
![]()
- Functional group isomerism: Isomers which differ in the functional group are called functional group isomers and the phenomenon is called functional group isomerism. This isomerism is shown by alcohols and ethers and aldehydes and ketones.
E.g. compound with the molecular formula C2H6O may be an alcohol ethanol (CH3-CH2OH) or an ether methoxy methane (CH3-O-CH3).
- Metamerism: Isomers which differ in the carbon chain (alkyl groups) around the functional group are called metamers and the phenomenon is called metamerism. It is commonly shown by ethers.
E.g.: Ether with molecular formula C5H12O may be methoxybutane (CH3-O-CH2-CH2-CH2-CH3) or ethoxypropane (CH3-CH2-O-CH2-CH2-CH3).
2. Stereo isomerism
Compounds having same molecular formula but different spatial arrangement of atoms are called stereoisomers and the phenomenon is called stereoisomerism. They have same atom to atom bond.
There are two types of stereo isomerism – Geometrical isomerism and Optical isomerism. The diagrammatic representation of different types of isomerism is:

 Ritan Sheth
Ritan Sheth
