- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
WATER POLLUTION
Presence of undesirable materials in water which is harmful for the human beings and plants is known as water pollution. Normal properties of the water can be changed by the presence of these foreign materials.
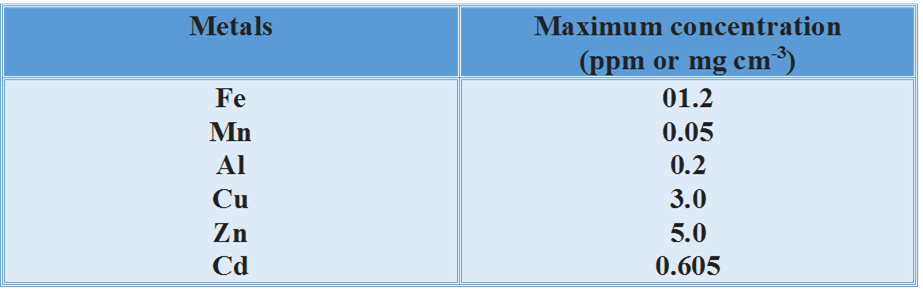
Maximum prescribed concentration of some metals in drinking water
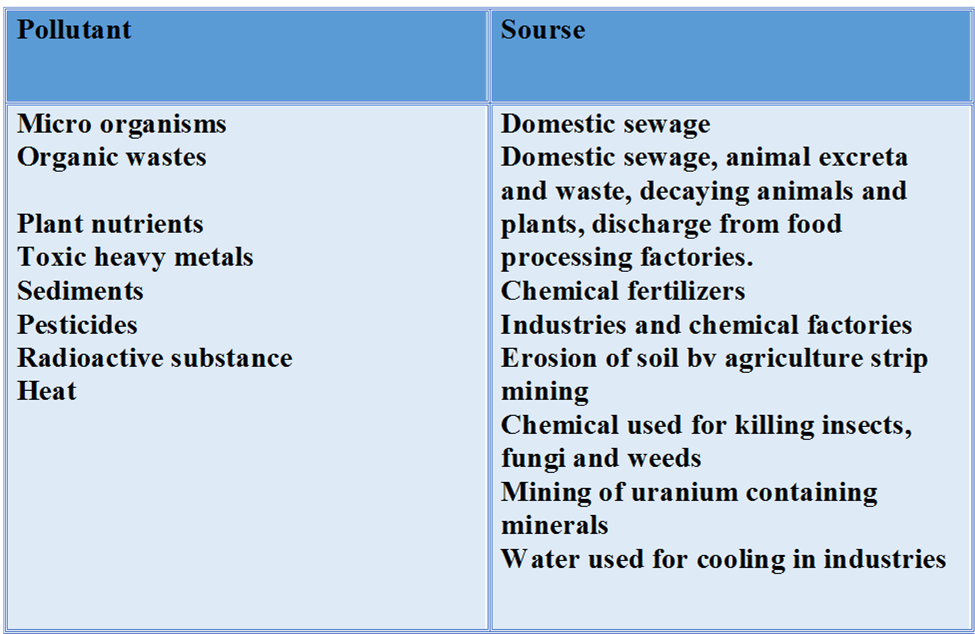
Major water pollutants
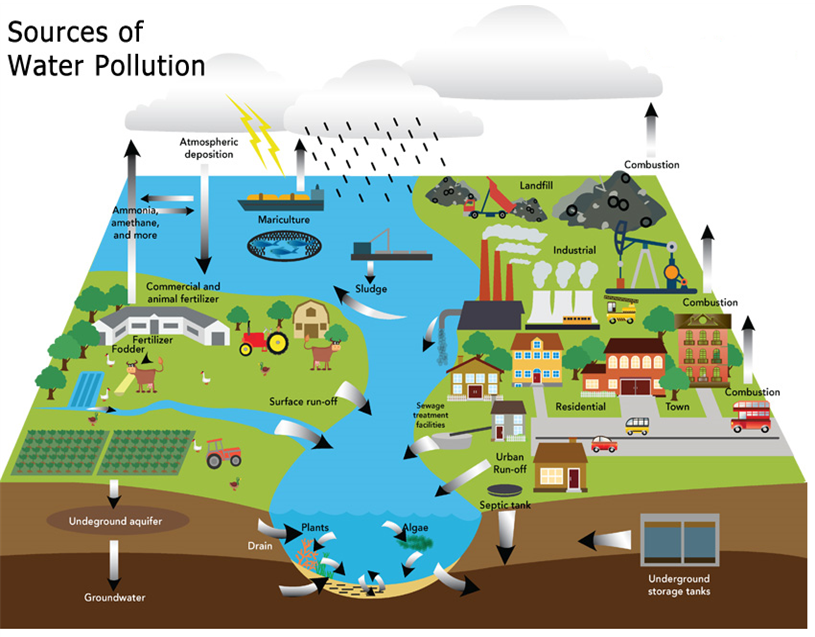
Causes of Water Pollution:
(i) Pathogens: Pathogens are the bacteria and the other organisms that enter water from domestic sewage and animal excreta.
Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis. It causes gastrointestinal diseases.
(ii) Organic Wastes: Organic matter such as leaves, grass, trash etc. can pollute water.
– Excessive growth of phytoplankton within water also pollute water.
– Large numbers of bacteria in water can consume oxygen dissolved in water by decomposing organic matter present in water.
– If the concentration of dissolved oxygen in water is below 6 ppm, the growth of fish gets inhibited.
– If too much of organic matter is added to water, all the available oxygen is used up. This can cause the death of the aquatic life.
• BOD (Biochemical Oxygen Demand)
It is defined as the amount of oxygen required by bacteria for the breakdown of the organic matter present in a certain volume of a sample of water.
The amount of BOD in water is a measure of the amount of organic material in the water. Clean water has BOD value of less than 5 ppm.
Highly polluted water could have a BOD value of 17 ppm or more.
• Chemical Pollutants
(i) Industrial Wastes: Chemical reactions carried in the industrial units also pollute water to a great extent. For example, lead, mercury, nickel, cobalt etc. These chemicals give very bad effect to the groundwater and waterbodies are polluted due’ to the chemical reactions known as leaching.
Organic chemicals like petroleum products also pollute many sources of water e.g., major oil spills in oceans.
(ii) Pesticides: These are mostly chlorinated hydrocarbons, organophosphates and metallic salts etc. They dissolve in water to small extent and pollute it. Since all the pesticides are toxic in nature, they are injurious to both plants and animals.
(iii) Polychlorinated biphenyls (PCBS): These are the chemical compounds used as fluids in transformers and capacitors. These are released in atmosphere as vapours. They mix with rain water and thus contaminate the water.
(iv) Eutrophication: The process in which algae like organisms reduce dissolved oxygen in water is called as eutrophication. It is harmful for aquatic life.
• International Standards for Drinking Water
Fluoride: Concentration of fluoride up to 1 ppm or 1 mg dm-3, is not harmful for human
beings if it is used as drinking water. The F~ ions make the enamel on teeth much harder by converting hydroxyapatite [3Ca3(P04)2- Ca(OH)2] the enamel on the surface of the teeth, into much harder fluorapatite, [3Ca3(P04)2- CaF2]. Concentration of F~ above 2 ppm causes brown mottling of teeth. Excess of fluoride is harmful to bones also.
Lead: Upper limit concentration of lead in drinking water is about 50 ppm. Lead can damage kidney, lever, reproductive system etc.
Sulphate: At moderate level it is harmless but excess is harmful.
Nitrate: The maximum limit of nitrate should be 50 ppm. Excess nitrate in drinking water can cause diseases such as methemoglobinemia (blue baby syndrome).
Chemical Oxygen Demand (COD): Water is treated with K2Cr207 in acidic medium to oxidise polluting substance which cannot be oxidised by microbial oxidation. The remaining is determined by back titration with suitable reducing agent.
From the concentration of K2Cr207 consumed, the amount of O2 used in the oxidation is calculated.
K2Cr2O7(aq) + 4H2SO4(aq) → K2SO4(aq) + Cr2(SO4)3(aq) + 3H2O + 3O

 Ritan Sheth
Ritan Sheth
