- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
STRUCTURAL REPRESENTATION OF ORGANIC COMPOUNDS
An organic compound can be represented by the following ways:
Complete structural formula:
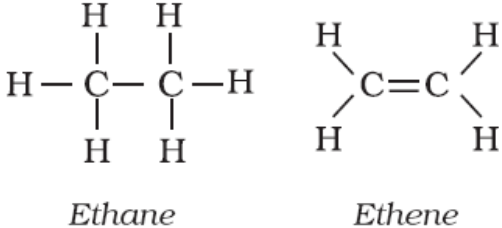
Here all the bonds between atoms are denoted by dashes ( ). A single dash represents a single bond, a double dash represents a double bond and a triple dash represents a triple bond. E.g.
Condensed stuctural formula: Here the carbon-hydrogen bonds or all the bonds are omitted except the multiple bonds. It is a simplified representation of an organic compound.
E.g. ethane – CH3CH3, propane – CH3CH2CH3, butane – CH3CH2CH2CH3, ethene – CH2=CH2 etc.
The condensed formula can again simplified as follows:
Butane – CH3(CH2)2CH3, Hexane- CH3(CH2)4CH3, Decane – CH3(CH2)8CH3 etc.
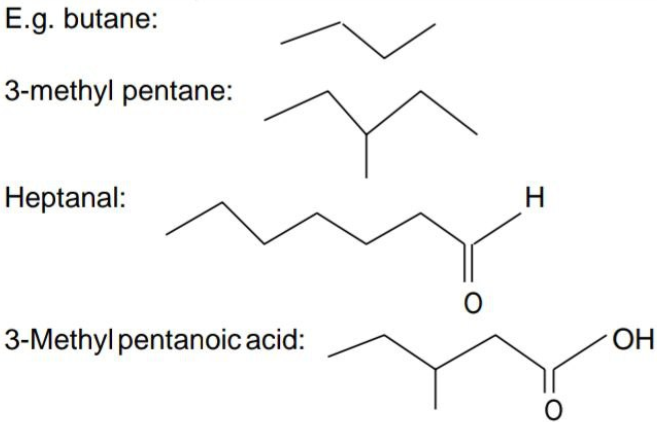
Bond line representation: It is the simplest form of representation of an organic compound. Here carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The free terminals denote methyl (–CH3) groups.
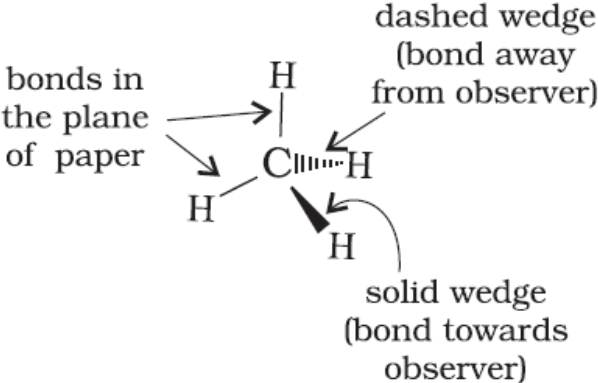
Three-Dimensional Representation (Wedge Representation): Here the structure of an organic molecule can be represented by using solid (![]() ) and dashed (
) and dashed ( ![]() ) wedges.
) wedges.
1. The solid-wedge is used to indicate a bond projecting out of the plane of paper, towards the observer.
2. The dashed-wedge indicates the bond projecting out of the plane of the paper and away from the observer.
3. The broad end of the wedge is always towards the observer. The bonds lying in plane of the paper are depicted by using a normal line (—).
E.g. methane

 Ritan Sheth
Ritan Sheth
