cyclic and non cyclic photophosphorylation and The Electron Transport
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Cyclic and Non cyclic photophosphorylation
Cyclic photophosphorylation :
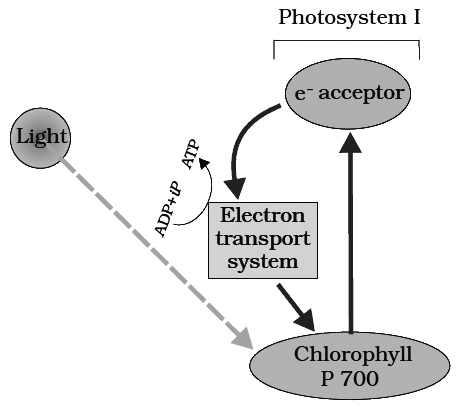
The process of cyclic photophosphorylation involves only photosystem I.
A photon of light excites an electron of chlorophyll a molecule (of PS I) and the excitation energy is eventually passed on to the reaction centre P700.
This excited electron is expelled from the chlorophyll molecule and is accepted by an electron acceptor Fe-S.
By initiating this electron transfer chain, P700 molecule becomes oxidised.
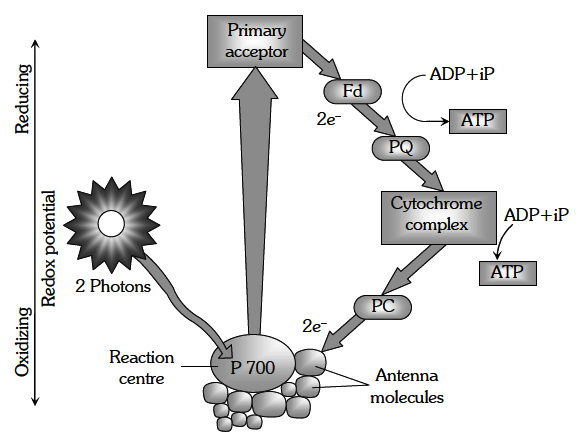
Fe-S passes this electron to other acceptors in the following sequences : Ferredoxin, Plastoquinone, Cytochrome b6-f complex and plastocyanin.
The oxidised P700 serves as a natural electron acceptor from plastocyanin. All the above electron acceptors are in oxidised state before accepting electrons (therefore these act as electron acceptors) and get reduced after accepting the electron.
As the electron is transferred from one acceptor to another, certain amount of energy is released. This energy is used to generate A TP (while passing between Fd and PQ and/or PQ and cyt. b6-f complex).
When only PSI is functional, the electron is circulated within the photosystem and the phosphorylation occurs due to cyclic flow of electrons.
A possible location on where it occurs is in the stroma lamellae.
The stroma lamellae membrane lacks PSII as well as NADP reductase enzyme.
The excited electron does not pass on to NADP+ but is cycled back to the PSI complex through electron transport chain.
The cyclic flow hence, results only in the synthesis of ATP, but not of NADPH + H+.
Cyclic photo phosphorylation also occurs when only light of wavelengths beyond 680 nm are available for excitation.
(b) Non-cyclic photophosphorylation:
It involves photosystem I as well as photosystem II. Both these systems absorb light photons. For convenience, the non-cyclic photophosphorylation is described here in following steps:
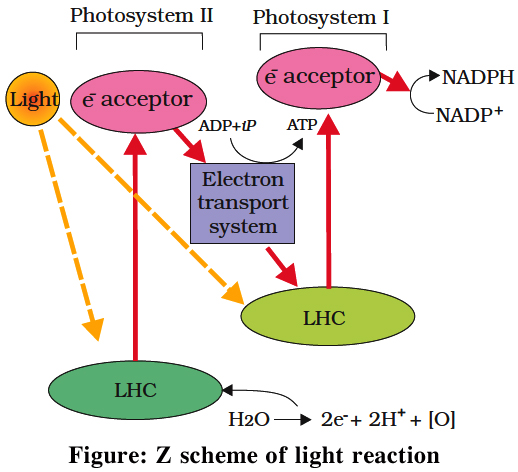
(i) When P680 absorbs a photon of red light, it gives out an electron, and thus becomes positively charged. This electron from P680 passes to P700 through a series of electron carriers, like Phaeophytin (Primary acceptor), plastoquinone, cytochrome b6-f complex, plastocyanin.
This movement of electron is downhill in terms of redox potential scale.
In this series, enough energy is released when the electron is transferred from PQ to cytochrome b6-f complex. It is utilized to synthesize ATP.
(ii) Simultaneously, electron in the reaction centre of PS I also get excited when they receive red light of 700 nm and these electrons are transferred to another receptor having high redox potential. These electrons then move downhill again, this time to a molecule of NADP+. This addition of electron and a proton, reduce it to NADPH + H+.
Differences between Cyclic and Non-cyclic Photophosphorylation

different types of transport
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Different types of transport
Plants show transport of various substances over short distances and long distances.
Sport-distance transport of substances occurs through diffusion and cytoplasmic streaming supplemented by active transport.
Long distance transport of materials occurs through vascular system i.e. xylem and phloem. This is also known as translocation. The direction of translocation is essentially unidirectional in case of water. It is multidirectional in case of minerals and organic solutes.
Transport of materials into and out of the cell is performed by number of methods like (I) Diffusion, (II) Facilitated diffusion and (III) Active transport.
Diffusion
The movement of molecules or ions from the region of higher concentration to the region of lower concentration, until the molecules are evenly distributed throughout the available space is known as diffusion.
The diffusing particles create a certain pressure called as diffusion pressure (DP) which is directly proportional to the number or concentration of diffusing particles. The molecules move from higher DP to lower DP.
Characteristics of Diffusion
Following are the important characteristics of diffusion:
1.The molecules (or ions) diffuse from region of their higher concentration to region of their lower concentration (passive process). This continues till an equilibrium is established.
2.The diffusing molecules move randomly along concentration gradient.
3.The direction of diffusion of one substance is independent of the movement of another substance.
4.It is very important to plants since it is the only means for gaseous movement within the plant body.
Concept Builder
Factors affecting the rate of diffusion
1. Temperature. The rate of diffusion increases with increase in temperature. This is because free energy of molecules increases with rise in temperature.
2. Density of molecules. It shares inverse root relation. This can be deduced from Grahm's law of diffusion which states that the rate of diffusion of gases is inversely proportional to the square root of their densities (density = mass per unit volume).
r = ![]()
where, r = rate of diffusion and d = density
3. Medium in which diffusion occurs. The rate at diffusion would be slower if the medium is concentrated, i.e., increase in the number of foreign molecules causes the rate of diffusion to decrease. Thus a gas would diffuse more rapidly in vacuum than in air.
4. Diffusion pressure gradient (DPG). It is the difference in the concentration of the diffusing molecules between one area and another over a specific distance. The steeper is the diffusion, pressure gradient, the faster is the rate of diffusion.
I. Facilitated diffusion
The diffusion of hydrophilic substances along the concentration gradient through fixed membrane transport protein without energy involvement is called facilitated diffusion.
The diffusion of any substance across a membrane also depends on its solubility in lipids, the major constituent of the membrane.
Substances soluble in lipids diffuse through the membrane faster.
Facilitated diffusion cannot cause net transport of molecules from a low to a high concentration, this would require input of energy.
Transport rate reaches a maximum when all of the protein transporters are being used (saturation).
It is very specific. It is sensitive to inhibitors which react with protein side chains.
The porins form huge pores in the outer membranes of the plastids, mitochondria and some bacteria allowing molecules upto the size of small proteins to pass through.
Water channels are made up of eight different types of aquaporins.
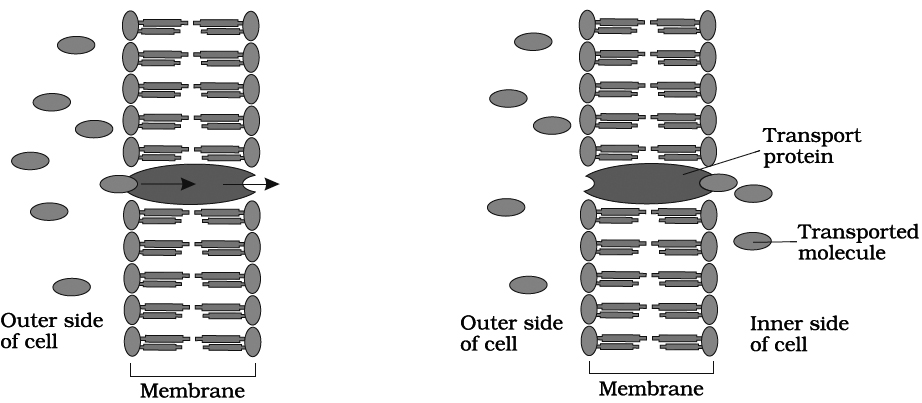
Passive symports and antiports
a.Uniport : When a molecule moves across a membrane independent of other molecules.
a.Cotransport: When two types of molecules move together with the help of carrier protein. It is of two types:
(i) Symport: Both molecules cross the membrane in the same direction at the same time.
(ii) Antiport: Both molecules move in opposite direction.
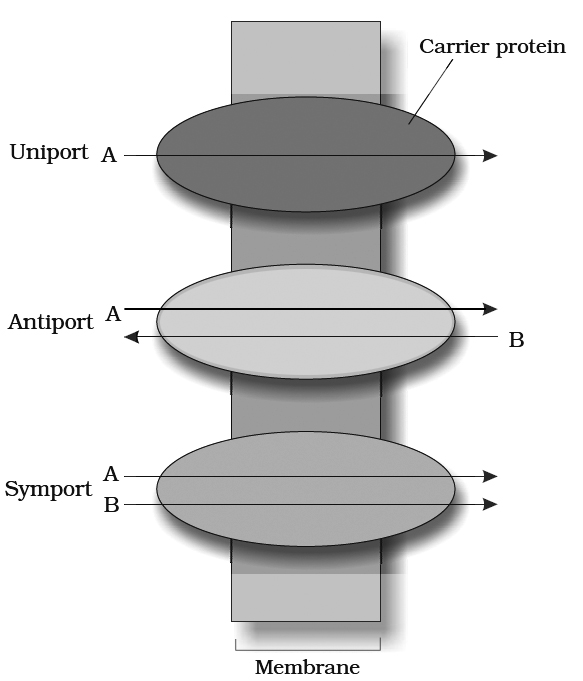
III. Active transport
Transport of materials across a membrane with the help of mobile carrier protein and ATP.
It is an uphill transport i.e., against concentration gradient and is faster than passive transport.
The rate of active transport reaches a maximum when all the protein pumps have been used in transport this is called saturation effect.
Carrier proteins are highly specific like enzymes.
They are also sensitive to inhibitors that react with protein side chains.
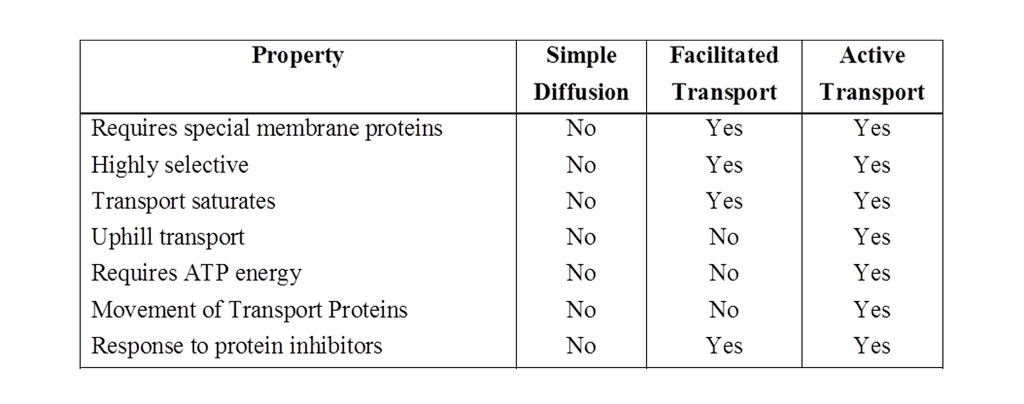
plant water relations
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Plant water relation
Membrane permeability
Permeability is the degree of diffusion of gases, liquids and dissolved substances through a membrane.
Normally, permeability of a given membrane to a particular substance remains unchanged.
However, changes in permeability can be brought about by many artificial and natural changes in the environment.
Following four types of membranes have been recognized on the basis of permeability:
1.Permeable. This type of membrane allows a free diffusion of both solvent and solute or ions through them. e.g., plant cell wall i.e., cellulosic and lignified.
2.Impermeable. The membranes with deposits of cutin and suberin do not allow the entry of water, dissolved substances and gases, hence, called impermeable, e.g., cell wall with thick layer of cutin on its surface.
3.Semi-permeable. A membrane that is impermeable to solute molecules but is permeable to the solvent is called a semipermeable membrane. This forms a perfect partition between two osmometers. e.g., copper ferricyanide membrane, parchment membrane, cellophane, collodion membranes.
4.Selectively or differentially permeable membrane. This membrane allows some molecules and ions to enter readily, while allowing others more slowly and does not allow certain molecules at all (i.e., different substances diffuse at different rates). Biological membranes, particularly the cell membrane (plasmalemma), tonoplast (vacuolar membrane) and the membranes surrounding sub cellular organelles are selectively permeable.
A variety of biologi.cal phenomenon are used to explain plant water relations, like osmosis, turgor pressure, wall pressure, DPD, water potential and imbibition. These are described separately in detail.
I. Osmosis (Term by Nollet)
Osmosis is a diffusion of solvent molecules through a semipermeable membrane.
Osmosis may be defined as the passage of solvent molecules from a region of their higher concentration to a region of their lower concentration through a semipermeable membrane.
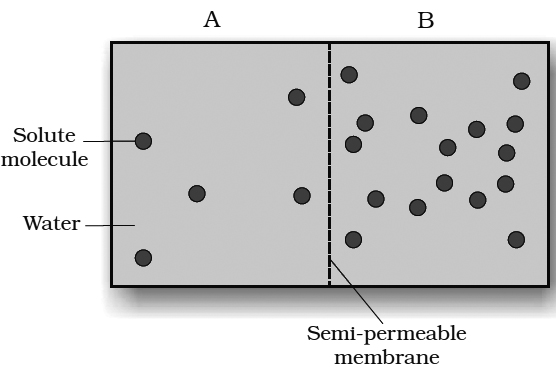
Osmosis can be demonstrated by a simple experiment in laboratory.
Animal bladder or parchment membrane is tied to the wide mouth of a thistle funnel.
Concentrated sugar solution or syrup is filled in the tube of the thistle funnel.
Now, the wide mouth of thistle funnel is immersed in a beaker containing water.
The level of the solution in the tube of the funnel is marked.
After sometime, the level in the tube increases.
This is due to the entry of water molecules from beaker into the thistle funnel.
The concentration of water molecules in the beaker is more than their concentration inside the thistle funnel.
Therefore, water molecules move from the region of their higher concentration (i.e., from beaker) to the region of their lower concentration (i.e, inside the funnel).
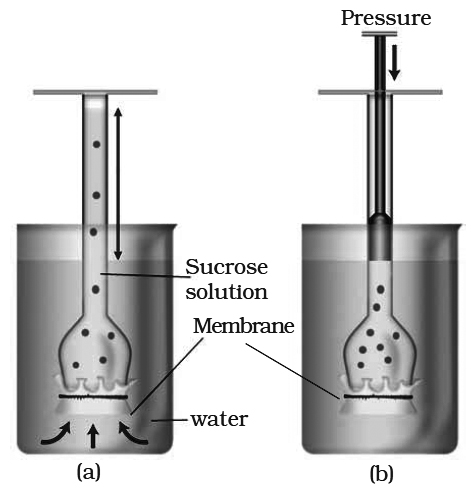
A thistle funnel is filled with sucrose solution and kept inverted in a beaker containing water. (a) Water will diffuse across the membrane (as shown by arrows) to raise the level of the solution in the funnel (b) Pressure can be applied as shown to stop the water movement into the funnel.
1.Endosmosis. When a cell is placed in a hypotonic solution water will enter into the cell from the outer (hypotonic) solution. It is because the cell sap is more concentrated (has less water molecules) than the outer solutions. This process of diffusion of water into the cell from outside is known as endosmosis. It will result in increase in the volume of the cell. e.g., Raisins placed in water.
2.Exosmosis. When a cell is immersed in hypertonic solution, water will diffuse out of the cell, because the concentration of water molecules in the cell is more than the outer solution. This process is described as exosmosis. e.g., grapes placed in sugar solution.
Osmotic Pressure (Term by Pfeffer)
It is the "maximum pressure which can develop in a osmotically active solution when it is separated from pure water by a semi-permeable membrane".
It is also defined as "the pressure needed to prevent the passage of pure water into an aqueous solution through a semipermeable membrane thereby preventing an increase in the volume of the solution".
The osmotic pressure (OP) depends upon (i) the concentration of solute particles, (ii) ionisation of solute particles, (iii) hydration of solute particles, (iv) temperature.
An increase in the condmtration of solutes in the solution increases the osmotic pressure.
If the solute ionises in solution, the number of particles increase, thus raising the osmotic pressure.
If solute molecules are hydrated, the water molecules bound with the solute are not able to diffuse and hence increase the osmotic pressure.
An increase in temperature raises the osmotic pressure of solution.
Plant cells exhibit a considerable range of variations in osmotic pressure.
In land plants, it varies from 5-30 atms. In aquatic plants, it varies from 1-3 atmp.
Plants of arid regions possess high OP.
Highest osmotic pressure is recorded in a halophytic plant, Atriplex confertifolia i.e., 202.5 atms.
OP of electrolyte is 2-3 times greater than a nonelectrolyte.
Osmotic pressure can be calculated by
OP(π ) = miRT [m =Molar concentration, i = Ionization constant, R = Gas constant,
T =Temperature (273°K)]
Osmotic pressure is numerically equal to osmotic / solute potential (Ψs) but has a positive value
Ψs = -π
Factor affecting OP
(1) Concentration of solute particles: OP of solution depends upon the concentration of solute in a given solvent. If the concentration of solute is increased then the OP of solution is also increased.
(2) Ionization of the solute molecule: OP of a solution depends upon the ionization of solute. Increased number of ions increases the OP.
(3) Temperature: OP of solution increases with increase in temperature.
(4) Hydration: Hydrated solute molecules will decrease the number of free water molecules, thereby increasing OP.
Reverse Osmosis:
It is the reverse movement of water through a semipermable membrane from a more concentrated solution to a more dilute solution by applying external pressure on the more concentrated solution, the pressure required to bring about reverse osmosis is more than the one required to prevent osmotic entry of water into it.
The method pushes out pure water.
Reverse osmosis is used in chemical industries to obtain pure water and also to purify water for drinking purposes.
Role of osmosis
1.Plants absorb water by osmotic mechanism.
2.Movement and distribution of water across the cells occur by osmosis.
3.Opening and closing of stomata is affected by osmosis.
4.The resistance of plants to drought and frost increases with increase in osmotic pressure of their cells.
5.Growth of young cells is due to osmotic pressure and turgor pressure.
6.Maintenance of turgidity.
Types of Solutions
In relation to cell sap, solutions can be of following three types:
1.Hypertonic solution. A solution whose concentration is more than that of the cell sap is known as hypertonic. If a cell is placed in such a solution, water will diffuse out of it (exosmosis) and the protoplasm would contract or shrink.
2.Hypotonic solution. When the concentration of a solution is less than that of the cell sap, it is known as hypotonic. If a cell is immersed in hypotonic solution, water will diffuse into the cell (endosmosis) and it will increase in size.
3.Isotonic solution. A solution with concentration equal to that of cell sap, is known as isotonic. If a cell is placed in isotonic solution there would be no net diffusion of water. As a result there is no change in the volume and weight of the cell.(Neither endosmosis nor exosmosis).
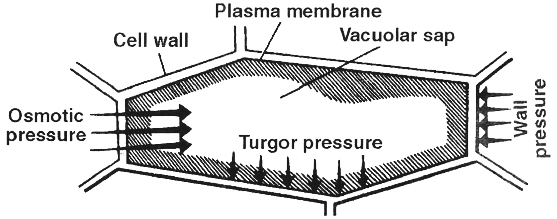
II. Turgor Pressure (TP)
It is a pressure which is developed in an osmotic system due to entry of water.
It causes swelling of the system.
Protoplasm of a plant cell functions as an osmotic system such that on absorption of water it becomes turgid and the turgid protoplast presses the cell wall towards the outside with a force called turgor pressure.
1. The cell wall also exerts a pressure over the protoplast, it is called wall pressure (WP).
2. Normally wall pressure is equal but opposite to turgor pressure (WP = TP).
3. Turgor pressure and wall pressure are positive pressures (negative TP is characteristic of plasmolysed cell and xylem vessels).
4. Reduced turgor pressure results in loss of turgidity.
5. Turgor change is also responsible for different types of plant movements and also for stomatal movement.
III. Diffusion Pressure Deficit (DPD) (term by Meyer) or
Suction Pressure (SP) (term by Renner)
Each liquid has a specific diffusion pressure.
Pure water has the maximum diffusion pressure.
The solution prepared by dissolving solute (such as sugar or salt) in pure water has lesser diffusion pressure as compared to pure solvent or water (though the solution has higher osmotic pressure).
In this way, there is always a difference between the diffusion pressure of solvent and its solution. Therefore, diffusion pressure deficit (DPD) may be defined as the difference between the diffusion pressure of a solution and a pure solvent, when both are subjected to the same atmospheric pressure.
To remove this deficit, the solution would absorb more solvent molecules, means water moves from low DPD to high DPD.
In this way, diffusion pressure deficit is the water absorption capacity of a solution.
Therefore, DPD can also be called as suction pressure. Its value is always positive for a cell.
Diffusion Pressure Deficit = Osmotic pressure - Turgor pressure
i.e., DPD = OP - TP
The above relationship indicates, that
1.When a cell is flaccid, its DPD = OP.
2.The entry of water into the cell causes development of TP.
3.When the increasing turgor pressure becomes equal to that of decreasing osmotic pressure, the entry of water into the cell would stop. This is called turgid condition of the cell. This can be expressed as
OP - TP = 0, thus DPD = 0
Concept of Water Potential
Water moves from region of higher free energy to that of lower free energy.
Thus, the net movement of water molecules occurc down the energy gradient.
The free energy of solvent can be increased by increasing the temperature and pressure.
The difference between the free energy of water molecules in pure water and the free energy of water in any other system (e.g., water in the solution or in a cell) is termed the water potential of that system.
water transport
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Long Distance Transport Of Water
The amount of water that can be held by soil, depends upon the total pore space in the soil.
Water is present in the spaces between the soil.
The total amount of water present in soil is called Holard.
The water available to plants is Chresard.
The rest of soil water is called Echard.
Concept Builder
Various types of ground water are:
(i) Run off water : Water which flows along the surface of soil and reaches to the nearest water body constitute run off water. This water is not available to the plants.
(ii) Gravitational water: Water Which percolates down through the soil macropores (20-50mm diameter) under the influence of gravity and reaches the water table is called gravitational water. This type of water is also not available to the plants.
(iii) Capillary water: Water retained by soil micropores. (£ 20 mm diameter) in the form of fine capillaries constitutes capillary water. This type of water is available to the plants.
(iv) Hygroscopic water: Water held in form of a very thin film around the soil particles by the forces of absorption constitutes hygroscopic water. This type of water is not available to the plant.(v) Chemicaliy combined water: Water which combines with inorganic salts of the soil in the form of water of hydration constitutes chemically combined water. This type of water is also not available to the plants.
(vi) Water vapours: They are present in soil air spaces, Normally, they are not available to the plants. Under certain conditions they are useful in the phenomenon of night recovery.
Water Absorption and Pathway of Water Across the Root
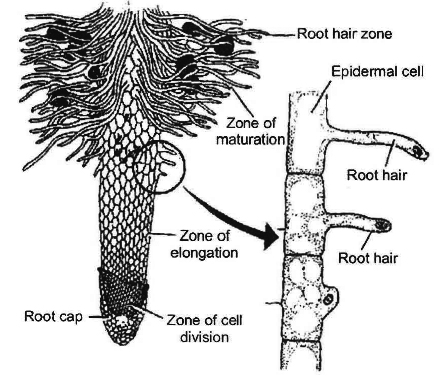
Water absorbing structure of the plant is root hair zone. Root hair is tubular prolongation of epiblema cells.
Root hairs are unicellular, short lived and arranged in an acropetal manner.
Root hairs are found in zone of cell maturation.
During transplantation the root hairs are removed, that is why, the plant remains wilted in the new habitat.
The cell wall of root hair is made up of two layers.
The outer wall is of pectin which dissolves in water, so that root hair surface becomes slimy and sticky.
The inner wall is made up of cellulose.
They are about 10 µm in diameter.
There OP is higher (3 - 8 atm) as compared to soil solution (less than 1 atm.
Many forest trees, shrubs and some conifers have scanty root hairs so they make association with the fungi, called mycorrhiza.
Orchid roots have a specific type of tissue for absorbing environmental moisture, this tissue is called velaman tissue.
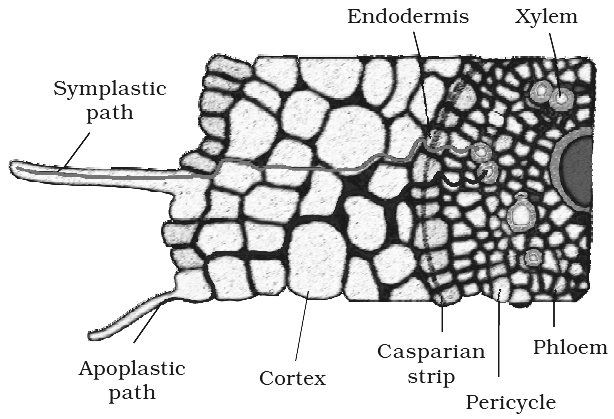
Movement of water from root hair cell to xylem may occur by two possible paths:
1. Apoplast Pathway: In this method, water passes from root hair cell to xylem through the walls of intervening cells without crossing any membrane or cytoplasm. The apoplastic movement of water beyond/ cortex is blocked due to the presence of casparian strips in the endodermal cells. Major movement of water through cortical cells occurs by this method, as cortical cells offer least resistance.
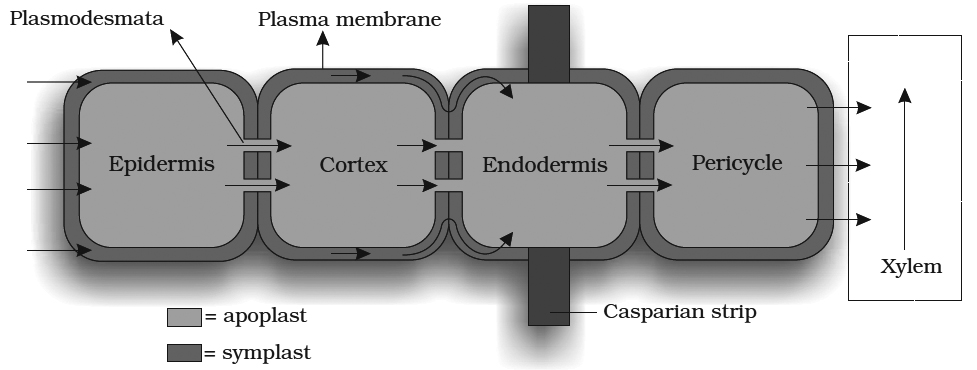
2. Symplast Pathway: In this method, water passes from cell to cell by crossing plasma membrane, therefore it is also known as transmembrane pathway. This may occur by two methods:
(i) Non Vacuolar Symplast Pathway: In this method, water passes between adjacent cells through plasmodesmata. It does not enter into the vacuoles.
(ii) Vacuolar Symplast Pathway: In this method, water crosses the tonoplast, surrounding the vacuole. This pathway offers a lot of resistance.
Beyond cortex (through endodermis and pericycle ) water is forced to move through symplast pathway. Terms Apoplast and Symplast were proposed by "Munch".
Mechanism of Water Absorption
(1) Passive absorption of water:
In actively transpiring plants, absorption of water takes place due to the forces developed at the transpiring surface of the plant (i.e., transpiration pull).
In this type, the cells of the root do not play any part, and it does not consume energy, hence it is known as passive absorption.
Thus in passive absorption, water is just pulled through the roots.
This is the most common (96%) and rapid method of water absorption.
Generally water is absorbed by the root hairs when the osmotic concentration of their sap is high.
This is made possible by transpiration taking place in the aerial parts of the plant.
It continously removes water from the sap of the root hairs which, in turn, are in contact of the soil water.
In actively transpiring plants, water loss from mesophyll cells occurs and increases their osmotic concentration.
It also results in the increase of their DPD.
As a result, water from neighbouring cells enters in them by osmosis.
These cells in turn have now increased their osmotic concentration or lowered their water potential.
Hence, water enters into them by osmosis from other adjacent cells.
In this way, mesophyll cells draw water from one another along the suction pressure gradient or DPD till it reaches the xylem of the leaf.
Once water is drawn from xylem of the leaf, the entire water column in the xylem of the leaf, stem and the root is lifted.
The movement of water is apoplastic.
In this way water is absorbed by the root hair due to diffusion pressure deficit gradient produced by transpiration that develops in the leaf.
Root simply acts as a path of water.
(2) Active absorption of water:
Although a very small amount of water (4%) is absorbed by active mechanism, it involves an expenditure of metabolic energy which comes from the respiring cells of the root.
Roots are actively involved in this method, so it is absorption by the roots.
Active absorption of water may occur in one of the two ways-(i) osmotic, (ii) non-osmotic.
Water absorption from soil and its inward movement is OP dependent or independent (OP of root hairs is higher than soil solution, OP of cortical cells is higher than root hairs).
Passage of water from living cells to xylem channel requires accumulation of solute in xylem which is an energy dependent process.
Hence, pumping of water in xylem channel is active.
This creates a positive pressure in xylem called root pressure.
Certain evidences also favour non osmotic absorption of water, requiring energy.
Difference between Passive and Active Absorption

Factors affecting water absorption
(1) Available soil water. Absorption of water is more, if the amount of available water is more. Rate of water absorption decreases, if the amount of soil water is below permanent wilting percentage or beyond field capacity.
(2) Soil air. Absorption of water takes place at a rapid rate in well aerated soil. Oxygen deficiency retards the growth of roots, thus inhibiting absorption of water. In the soil, if all the air spaces are filled with water the condition is known as water logging of soil. Such soil is physiologically dry soil.
(3) Concentration of soil solution. If the soil solution is highly concentrated due to the presence of salts, it will inhibit the water absorption. It is also a kind of physiological dryness.
(4) Soil temperature. An increase in soil temperature upto about 30°C favours water absorption. At higher temperatures water absorption is decreased and at O°C it is almost checked.
ASCENT OF SAP
Upward conduction of water in the form of dilute solution of mineral ions from roots to aerial parts is called ascent of sap.
Xylem is responsible for ascent of sap which can be proved by girdling experiment.
Concept Builder
Girdling or ringing experiment
Ringing expedmentwas first introduced by Malpighi (father of microscopic anatomy).
It consists of removing a ring of bark, i.e., all the tissues outside vascular cambium.
During Girdling or ringing experiment, two s mall twigs are taken.
Girdle or a ring of bark is removed from one of these twigs by a sharp knife.
In second twig, xylem is removed carefully without causing much injury to the bark or xylem can be blocked with wax.
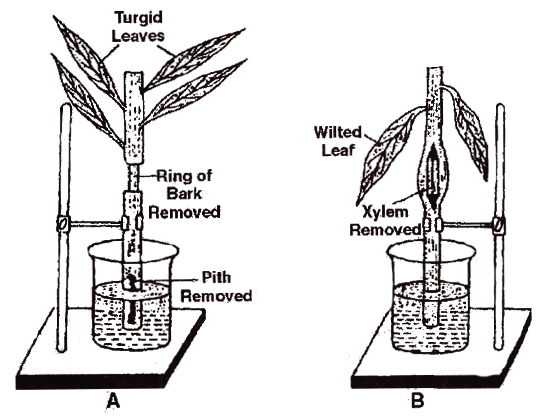
Determination of path of ascent of sap
A. Shoot with removed bark and leaves remains turgid.
B. Shoot with removed xylem Shows wilting of leaves.
Thus, the girdled part of the first twig contains only xylem and that of the second twig has all tissues except xylem.
Both the twigs we placed in separate beakers containing water.
After a period of time, leaves on the first twig appear turgid while those on the second/twig with no or blocked xylem, the leaves wilt.
This experiment shows that water conduction occurs through the xylem.
Mechanism of ascent of sap
Many theories were put forward to explain the mechanism of ascent of sap. These theories are placed in following three categories:
(1) Vital force theories
(2) Root pressure theory
(3) Physical force theories
Concept Builder
(1) Vital force theories
According to these theories, living cells are responsible for ascent of sap. Some of the important vital force theories are given below:
(a) Wastermaier concept: He suggested that the living component of xylem, i.e., xylem parenchyma is responsible for the conduction of water, while the tracheids and vessels act as a water reservoir.
(b) Godlewski's relay pump theory or clambering theory : According to this theory, conduction of water occurs due to activity of xylem parenchyma and medullary rays. It is believed that some rhythmic change occurs in the osmotic pressure of these cells. When their osmotic pressure is high, water is absorbed from the surrounding vessels, so the turgor pressure of these cells increases. Hence; the water is pumped to next level of xylem vessels, in a stair case like manner.
(c) Sir J.C. Bose's pulsation theory: He experimented on Indian telegraph plant (Desmodium gyrans). By the help of electric probe, he believed that the pulsation movement occurs in the cortex cells which are present just outside the endodermis, which is responsible for ascent of sap, but such pulsations were not found to be of universal occurrence.
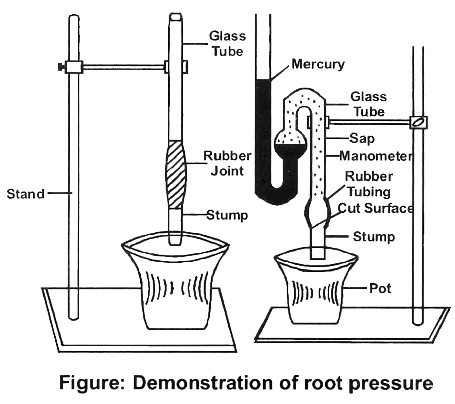
(2) Root pressure theory
When a potted plant is cut below the first leaf and manometer filled with water is attached on cut stem, the level of water in manometer increases.
It indicates that water is being pushed by roots after it is absorbed.
If a stem is cut near its base or incision is mad e into a plant, xylem sap is seen to flow out.
This phenomenon is known as exudation or bleeding.
Priestley stated Stand that the upward flow of water in bleeding is due to root pressure.
Stephan Hales coined the term root pressure and described it as "The hydrostatic pressure developed in roots due to accumulation of water absorbed by the roots".
The development of root pressure is the result of an active absorption.
This depends upon the active accumulation of solute in xylem sap.
This can be inhibited using cyanide, lack of O2 and low temperature.
(1) Root pressure can generate pressure of about 2 atm which is insufficient to raise the water up in tall trees.
(2) In some plants like conifers, root pressure has never been observed.
(3) In temperate regions, root pressure is generally low du ring summer when the rate of transpiration is high, in comparison to absorption.
(4) Ascent of sap continues even in the absence of root pressure.
(5) The greatest contribution of root pressure may be to re-establish the continuous chain of water molecules in the xylem, which often break under the enormous tensions created by transpiration.
(3) Physical force theories
According to the proponents of physical force theories, living cells do not take part in the translocation of water, but is brought about by some physical forces developing in the dead cells.
Some of the physical force theories are described in brief:
(a) Capillarity theory: According to Boehm, water moves upward partly due to capillaries of the vessel elements and partly due to atmospheric pressure.
The theory could not be accepted because of the following points:
(i) For capillary action, the capillary should be in contact with the free water surface, however, in plants, xylem is not in direct contact with the soil water.
(ii) Capillary action can be observed only in narrow vessels, but tall plants usually have wider vessels (0.03 mm diameter).
(b) Imbibition theory: According to Sachs, water moves upward due to imbibitional force between the cell wall of the xylem and not through the lumen of xylem vessels.
(c) Cohesive force and transpiration pull theory or Cohesion tension theory : This is the most widely accepted explanation for ascent of sap. It was proposed by Dixon and Jolly (1894). The theory is based essentially upon following three facts
(i) Cohesive force or tensile strength of water. The water molecules have a strong mutual attraction, i.e., they tend to 'stick' to each other. This is called cohesion. They also tend to stick to the lignocellulosic wall of the xylem elements; this is called adhesion. A high cohesion of water molecules means that a relatively large tension is required to break a column of water. The magnitude of tensile strength of water is 10-30 MPa.
(ii) Continuity of water column. Water forms a continuous column from the leaves to the roots. The cohesive and adhesive forces are very great and do not allow the water column to break or pull away from the walls of the xylem.
(iii) Transpiration pull. Water evaporates from mesophyll cells of the leaf due to transpiration. This results in an increase in their diffusion pressure deficit (DPD) or suction pressure. Since the water in the mesophyll cells is in contact with xylem sap of stem and roots through tracheids in the veins, the diffusion pressure gradient gradually passes down to the xylem of the root (negative pressure) and water is pulled up. Thus due to transpiration, there is a constant pull or tension on water column in upward direction. This is called transpiration pull or tension in the water column of xylem due to transpiration.
Water potential as low as (-3 MPa or -30 bars) has been measured in the leaves borne on tree tops.
This can overcome gravitational pull and resistance offered by the capillaries of xylem vessels.
The theory assumes tracheids to be more efficient than vessels.
They believed that partition walls of the tracheids confer stability on the stressed transpiration stream.
transpiration
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
TRANSPIRATION
Transpiration is the loss of water in the form of vapours from the aerial parts of the plant.
The loss of water is no great that it reduces water level in the soil and can lead to e ea of plant, but transpiration is said to be necessary for water and mineral absorption, ascent of sap and lowering the temperature (cooling effect).
So, transpiration is called as necessary evil (Curtis) or an unavoidable evil (Steward).
About 98% of water absorbed by land plants evaporate from their aerial parts and is lost into the atmosphere.
Less than 1 % of the water reaching the leaves is used in photosynthesis and plant growth.
Types of Transpiration
Based on the plant parts or structure involved, following four types can be recognised:
1. Stomatal transpiration: It is the transpiration that occurs through the stomata. The epidermis of leaves and green stems have numerous stomata. These are responsible for about 50 -97% of the total water transpired.
2. Cuticular transpiration : Water vapours are also lost directly from the outer walls of the epidermal cells through the cuticle. Cuticle is a wax like layer of cutin that covers the epidermis of leaves and stems. It reduces the water loss but may give out water vapours through the cracks. It commonly constitutes 3-10% of total transpiration. It is maximum upto 50% in herbaceous plants, ferns etc. growing in shady places.
3. Lenticular transpiration : Lenticels are aerating pores in the cork of the woody stems, twigs and fruits. Water vapours are lost through these openings. The amount of water vapours lost through lenticels is usually insignificant (approximately 0.1% of the total water loss).
4. Bark transpiration: This occurs through the bark of woody stem. It contributes about 1% of the total transpiration.
Structure of Stomata
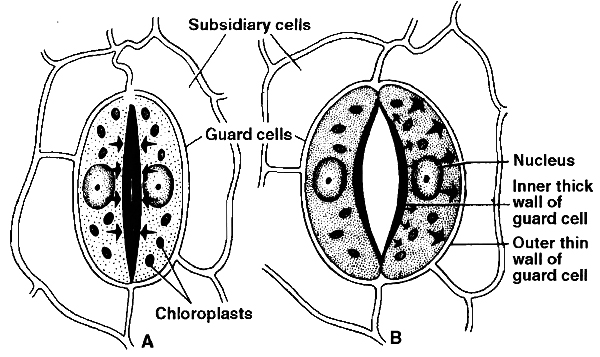
Stomata are the tiny apertures found on the epidermis of leaves and young green stems.
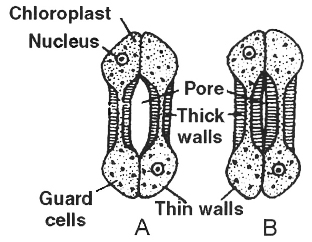
Each stoma is surrounded by two specialized epidermal cells, called guard cells.
They differ from epidermal cells in their shape (kidney or bean shaped) and in the presence of chloroplasts.
The inner wall of the guard cell is thick and elastic, whereas the outer wall is thin.
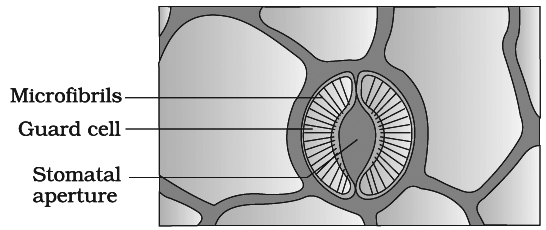
Opening of the stoma is also aided due to the orientation of the microfibrils in the cell walls of the guard cells.
Cellulose microfibrils are oriented radially rather than longitudinally, making it easier for the stoma to open.
Guard cells are bordered by one or more modified epidermal cells called subsidiary cells or accessory cells.
In monocots, guard cells are ellipsoidal or dumb-bell shaped (called graminaeous stomata or poaceous stomata).
These stomata have thin end walls and thick walled middle region.
The leaf surface, depending upon the species may contain 1000 to 60,000 stomata per square centimeter.
The total pore area is approximately 1 -2% of total leaf area.
Classification of stomata on the basis of their distribution
With the exception of few submerged hydrophytes, stomata are widely distributed amongst angiosperms and gymnosperms.
Based on their distribution on leaf surface, stomata are grouped in the following five categories:
1. Apple or mulberry type: Stomata are present only on the lower leaf surface, e.g., apple. Such leaves are called hypostomatic.
2. Potato type : The stomata occur on both the leaf surfaces, being more on the lower surface than that on the upper, e.g., potato, tomato, brinjal. Such leaves are called amphistomatic.
3. Oat type: The stomata occur equally on both the leaf surfaces, e.g., wheat, rice, grasses, etc. These leaves are also called amphistomatic.
4. Water lily type: The stomata are found only on the upper surface of the leaf, e.g., water lily. The leaves of such plants are found floating on water surface. These leaves are termed as epistomatic.
5. Potamogeton type: The stomata in some hydrophytes are either absent or vestigeal. Such leaves are called astomatic.
Concept Builder
Classification of Stomata on the basis of daily movement
Loftfield classified stomata into following four types on the basis of their daily movement:
(a) Alfalfa type. The stomata remain open throughout the day and closed throughout the night, e.g., pea, beans, radish, mustard, turnip, grapes etc.
(b) Potato type. The stomata remains open throughout the day and night except for a few hours in the evening e.g., onion, potatoes, cabbage, banana, etc.
(c) Barley type. The stomata open for only a few hours during the day and remain closed for rest of the period, e.g., barley, maize, wheat and other cereals.
(d) Equisetum type. In Equisetum (Horsetail), the stomata remain open throughout the day and night
Mechanism of Opening and Closing of Stomata
Stomata function as turgor operated valves.
When osmotic concentration of guard cells increases, water comes in and guard cells become turgid and stomata gets open.
Whenever, osmotic concentration of guard cells decreases water moves out, guard cells become flaccid and hence get closed.
This increase and decrease in osmotic concentration is explained by a number of theories, described below:
1. Photosynthetic Theory (Von Mohl and Schwendener) :
This theory proposes that in the morning, as soon as light is available chloroplasts of guard cell start photosynthesis, as a result sugars are produced, which increases the osmotic concentration of guard cells, water comes in, guard cells become turgid and stomata are open.
This theory is not accepted because photosynthetic activity of guard cell chloroplasts seems to be negligible and sugar does not occur in detectable quantity in guard cells (as Rubisco is absent in guard cell chloroplast).
2. Starch ![]() Sugar hypothesis (Classical theory).
Sugar hypothesis (Classical theory).
It was given by Sayre and Scarth and later modified by Steward.
This theory is called classical theory.
They indicated that interconversion of starch into sugar is determined by change in pH.
According to this theory, carbon dioxide liberated due to respiration is used in photosynthesis by mesophyll cells.
This results in increase in pH to 7-7.5.
In this alkaline state, starch is converted to glucose-1-phosphate.
In the dark, carbon dioxide accumulates in the intercellular spaces as it is not utilised in photosynthesis.
It lowers pH of the guard cells to about 5.
In this acidic state, glucose-1-phosophate is converted back to starch, leading to closure of stomata.
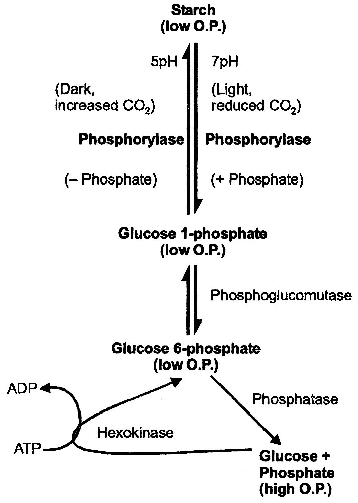
in guard cells according to Starch Sugar Hypothesis
Objectivess
(i) Starch is absent in onion.
(ii) Glucose does not appear in detectable quantity in guard cells.
3. Active K+ transport or Potassium pump theory.
Two Japanese scientists, S. Imamura and M. Fujino showed the accumulation of K+ in the guard cells during stomatal opening.
Later, Levitt (1974) explained the influx of K+ ions in the guard cells and their critical role in stomatal movement.
(a) Opening of stomata in light:
(i) In light, starch in the guard cells is incompletely oxidized into phosphoenol pyruvate (PEP). It is later converted into or anic acids, particularly malic acid. This reaction is catalyzed by an enzyme phosphoenol pyruvate carboxylase (PEPCO or PEPCase).
(ii) Malic acid dissociates into malate ion and protons (H+) in the guard cells.
(iii) H+ from guard cells, are transported to epidermal cells and K+ from epidermal cells gets into the guard cells through the agency of hydrogen-potassium ion exchange pump in the plasma membrane.
(iv) In the guard cells, K+ ions are balanced by malate anions. Besides, small amount of Cl– ions are also absorbed which neutralize a small percentage of K+ ions.
(v) The process of ion exchange requires ATP and thus, it is an active process.
(vi) Increased K+ and malate ions forms potassium malate and store it in vacuoles of the guard cells, increasing their osmotic concentration. Hence, water enters the guard cells by endosmosis.
(vii) Turgor pressure of the guard cells increases due to endosmosis and the stomata gets open.
(b) Closing of stomata in the dark:
(i) As CO2 is not utilized in photosynthesis during night, hence its concentration in the sub stomatal cavity increases.
(ii) An inhibitor hormone-abscissic acid (ABA) functions in the presence of CO2, It inhibits K+ ion uptake by changing the diffusion and permeability of the guard cells for positive ions.
(iii) The K+ ions are transported back to the epidermal or subsidiary cells from the guard cells.
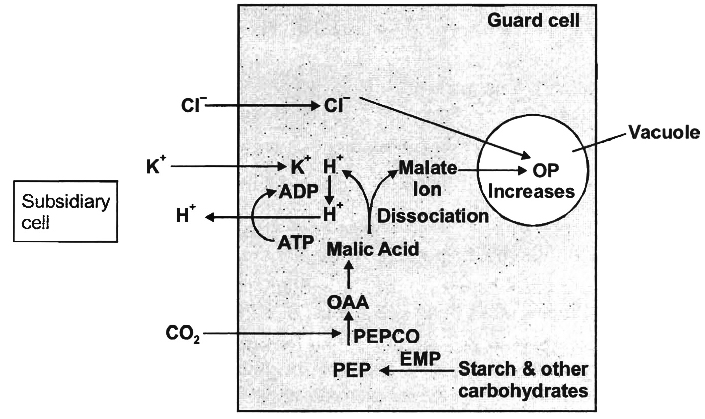
Concept Builder
In succulents stomata open during the night and close during the day. This is called scotoactive opening. These plants show night time formation of malic acid, e.g., Opuntia, Bryophyllum.
Factors affecting Transpiration
(A) External factors
(1) Light. Blue light induces maximum opening of stomata. In its absence, stomata remain closed. Light also affects the rate of transpiration by increasing temperature. Blue and red light are effective for transpiration, constituting its action spectrum.
(2) Relative humidity (vapour pressure gradient). In humid atmosphere (when the relative humidity is high) the rate of transpiration decreases. In dry atmosphere the relative humidity is low (low water vapour pressure), so the rate of transpiration increases.
(3) Temperature. Higher the temperature more is the rate of transpiration as it results in a higher vapour pressure gradient. Lowering of temperature decreases the rate of transpiration.
(4) Wind. If wind is not blowing, water vapours accumulate above the transpiring leaves which decrease the rate of transpiration. The blowing wind removes the accumulated humidity and brings fresh air capable of absorbing water (slow breeze) and thus, the rate of transpiration is enhanced.
Storm or wind of very high velocity causes closure of stomata, thus checking transpiration.
(5) Available soil water. If the available water in the soil is not sufficient, the rate of transpiration is decreased. A high concentration of salts in the soil water also reduces the rate of transpiration due to less water absorption.
(B) Plant Factors
1. Root-shoot ratio: Root absorbs water, hence if root shoot ratio decreases then transpiration decreases or vice versa. Short plants have a high root -shoot ratio.
2. Structure of leaf: Many features like thick cuticle, waxy coating, thick walled hypodermis, sunken stomata reduce the transpiration.
3. Number and distribution of open stomata, plant water, canopy structure are other factors affecting transpiration.
Significance of Transpiration
Transpiration has been described as a 'necessary evil' (Curtis) and an unavoidable evil (Steward). It is potentially harmful.
The stomata remain open for exchange of gases.
This also results in the loss of water as vapours.
Consequently, the water level in the soil is reduced, causing wilting of plants.
The stomata, however, cannot be closed to prevent water loss because this would also stop gaseous exchange needed for respiration and photosynthesis.
The advantages of transpiration are as follows:
(i) The absorption of water and ascent of sap to various parts of the plant body is mostly due to transpiration. Transpiration pull is responsible for mass movement of water and solutes in upward direction
(ii) Plants receive solar energy in very large amounts for the synthesis of carbohydrates. If there is no dissipation of energy, the temperature of leaf surface would rise to a lethal level in less than two minutes. Transpiration plays an important role here. It helps in dissipating this excess energy by evaporating water from the leaf surface and thus, helps in keeping the plant cool.
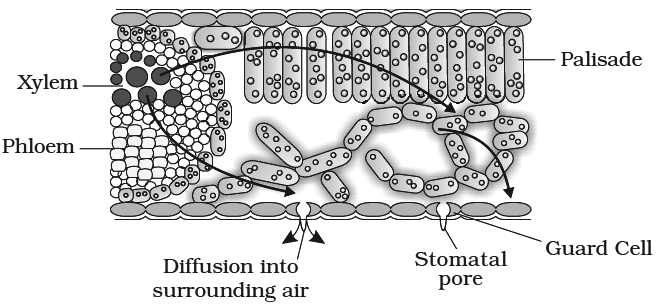
between the outside air and the air spaces of the leaf. The gradient is transmitted into the photosynthetic cells and on the water-filled xylem in the leaf vein.
(iii) Development of mechanical tissues, growth of root system, increasing ash and sugar content of fruits and development of resistance are other beneficial effects of transpiration.
Many chemicals (antitranspirants) have been found to reduce the rate of transpiration without affecting CO2 uptake e.g., Phenyl mercuric acetate (PMA, a fungicide), Abscisic acid (ABA) and CO2, Silicon emulsion and low viscosity waxes cover stomata as a film, allow CO2 and O2 exchange but resist diffusion of water.
Transpiration and Photosynthesis — a Compromis
Transpiration has more than one purpose; it
Creates transpiration pull for absorption and transport in plants.
Supplies water for photosynthesis.
Transports minerals from the soil to all parts of the plant
Cools leaf surfaces, sometimes 10 to 15 degrees, by evaporative cooling.
Maintains the shape and structure of the plants by keeping cells turgid.
An actively photosynthesising plant has an insatiable need for water.
Photosynthesis is limited by available water which can be swiftly depleted by transpiration.
The humidity of rainforests is largely due to this vast cycling of water from root to leaf to atmosphere and back to the soil.
The evolution of the C4 photosynthetic system is probably one of the strategies for maximising the availability of CO2 while minimising water loss.
C4 plants are twice as efficient as C3 plants in terms of fixing carbon (making sugar).
However, a C4 plant loses only half as much water as a C3 plant for the same amount of CO2 fixed.
uptake and transport of mineral nutrients
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Uptake and transport of mineral nutrients
Plants obtain their carbon and most of their oxygen from CO2 in the atmosphere.
However, they obtain remaining nutritional requirements from minerals and water (for hydrogen)
Unlike water, all minerals cannot be passively absorbed by the roots.
Two factors account for this: (i) minerals are present in the soil as charged particles (ions) which cannot move across cell membranes and (ii) the concentration of minerals in the soil is usually lower than the concentration of minerals in the root.
Therefore, most minerals must enter the root by active absorption into the cytoplasm of epidermal cells.
This needs energy in the form of ATP.
The active uptake of ions is partly responsible for the water potential gradient in roots, and therefore for the uptake of water by osmosis.
Some ions also move into the epidermal cells passively.
Ions are absorbed from the soil by both passive and active transport.
Specific proteins in the membranes of root hair cells actively pump ions from the soil into the cytoplasms of the epidermal cells.
Like all cells, the endodermal cells have many transport proteins embedded in their plasma membrane; they allow some solutes to cross the membrane, but not others.
Transport proteins of endodermal cells are control points, where a plant adjusts the quantity and types of solutes that reach the xylem.
Note that the root endodermis because of the layer of suberin has the ability to actively transport ions in one direction only.
transport by phloem
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Transport by phloem
Organic solutes such as glucose, sucrose produced during photosynthesis are translocated through phloem tissue.
The transport of photosynthates from the production centres (leaves) to the consumption centres (apices, roots, fruits, buds, tubers) through phloem is called translocation of organic solutes or long distance transport.
Translocation through phloem occurs in upward, downward and radial directions from the source (leaves) to the sink i.e., consumption centres.
Chemical analysis of the phloem sap revealed the presence of sugars upto 90%.
Sucrose constitutes 5-15% of the total sugars.
Other sugars like raffinose (triose), stachyose (tetrose) and verbascose (pentose) are also present in small quantities.
This analysis strongly suggests that phloem is the tissue concerned with the translocation of organic solutes.
Pure phloem sap may be collected by using sap sucking aphid
Concept Builder
Rabideau and Burr (1945) supplied C14O2 to a leaf during photosyinthesis (Tracer technique). Sugars synthesized in this leaf got labelled with 14C (tracer). Presence of labelled sugars (radioactivity) in the phloem showed that solutes are translocated through phloem.
Theories of Translocation of Organic Solute
1. Protoplasmic streaming hypothesis: This theory was proposed by a Dutch botanist, Hugo de Vries in 1885 and was supported by C.F. Curtis (1935). According to this theory:
(a) Protoplasm of the sieve tubes show continuous streaming from one end to the other.
(b) Organic solutes (sugars) which enter the sieve tube are passively carried by the streaming protoplasm from one end of the sieve tube to the other.
(c) Solutes move from one sieve tube to the next sieve tube by diffusion through the pores of the sieve plate. Thus, the streaming protoplasm acts as a conveyer belt or two-way escalator.
(d) Different substances move in different directions at the same time in the same sieve tube.
2. Pressure flow or mass flow hypothesis: This theory was proposed by Munch (1929) and elaborated by Crafts (1938). According to this theory, organic solutes are translocated "en masse" through the sieve tubes from the supplying end or source (leaves) to the consumption end or sink (roots, fruits, tubers).
(a) Mesophyll cells synthesize sugars during photosynthesis. As these get dissolved in cell sap, the osmotic concentration and DPD of mesophyll cells increases (Ψw decreases).
(b) Water enters the mesophyll cells from the xylem. Hence, the turgor pressure or pressure potential (Yp) of the mesophyll cells increases.
(c) Sugars dissolved in water move from mesophyll cells into the symplast system of sieve tubes through companion cells (Phloem loading).
(d) Solutes are carried "en masse" through the symplast to finally reach the consumption centres.
(e) At the consumption end, food materials (solutes) are either used up (as in roots) or are stored in an insoluble form (as in fruits, tubers). Hence, the osmotic concentration and, consequently, the turgor pressure in these cells will be low.
(f)Thus, a continuous turgor pressure gradient gets established across the symplast between the cells of the source and the cells of the sink.
(g) Water returns to the source (leaf) through the apoplast system
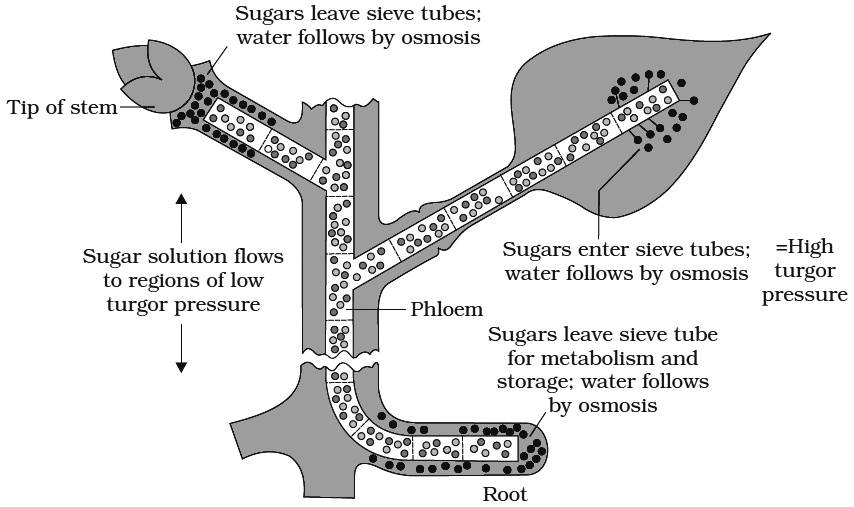
Objection to mass flow hypothesis :
(a) Bidirectional transport of organic solute in the same sieve tube needs explanation.
(b) Slime content and other fibrils of the sieve tube reduce the speed of flow of solutes even under high pressure.
(c) Mass flow is not a purely physical process as described by Munch because phloem cells utilised 0.1-0.5 percent of sucrose translocated through them. This is an evidence to show that phloem translocation (both loading and unloading) is an active process and requires metabolic energy.
Factors affecting Translocation of Solutes
Temperature:
Optimum temperature for translocation is 20°C and 30°C. The rate of translocation increases with increase in temperature. The temperature influences the root more than the shoot, since it acts as sink for the sugars.
Light:
The root/shoot dry weight ratio increases with increased light intensities. This indicates that translocation to root increases as compared to shoot when light intensity is increased.
Metabolic inhibitors:
The metabolic inhibitors can inhibit carbohydrate translocation. These include dinitrophenol (DNP), arsenite, azide, fluoride and hydrogen cyanide.
Mineral deficiencies:
The absorption and translocation of sucrose by a leaf is facilitated by boron. It helps sucrose to move easily through the cell membranes in the form of boron-sucrose complex.
Hormones:
Sucrose is much more efficiently translocated when growth regulators are applied such as kinetin, 1AA and gibberellic acid.
GUTTATION (Term by Burgerstein)
Plants growing under humid conditions in a moist warm soil often exhibit droplets of water along the margins of their leaves.
Phenomenon is commonly seen in Oat, Tomato, Cucumber, Garden Nasturtium and Saxifraga etc.
The loss of water in the form of liquid is called guttation.
In moist and humid conditions, the rate of absorption of water greatly exceeds transpiration.
The root pressure is built up which pushes the water up in the xylem ducts, from where it comes out on the leaf surface through special structures called hydathodes.
Hydathodes are present at the tips of veins in leaves.
A hydathode consists of a pore in the epidermis followed by large intercellular spaces and loosely arranged parenchyma called epithem and blindly ending xylem elements.
Guttated water contains inorganic and organic salts and is not pure.
Concept Builder
1.Osmotic pressure of 1 molar solution of a non-electrolyte would be 22.4 atmospheres at 0ºC.
2.Equimolar concentrations of two solutions of non-ionising substances will have same osmatic pressure.
3.The value of osmotic potential of an electrolyte will be greater by the degree of its dissociation into ions at a given temperature.
4.Plasmolysis can be demonstrated in epidermal peel of Rhoeo discolor leaf.
5.The auxin treated cells show increase in their metabolism. Respiration in these cells increases and more of energy is provided for the absorption of water.
6.At low temperature, water in the intercellular spaces freezes into ice, thus having higher OP. It causes exosmosis of water from cells causing desiccation.
7.If a fresh water plant is transferred to marine water, it dies due to exosmosis.
8.Root pressure is measured by manometer.
9.Stocking (1956) considered root pressure as an active process responsible for guttation and bleeding in plants.
10.Maximum root pressure recorded in plants is 2 bars which is sufficient to raise the water column to a height of 20 meter.
11.Root pressure is absent in gymnosperms.
12.Potometers are used for measuring/comparing the rates of transpiration.
13.Cobalt chloride paper method is also used to compare the rates of transpiration. Moisture coming out of stomata turns blue cobalt chloride paper to pink.
14.Porometers are used for assessing the total pore area (stoma).
15.Generally, stomata are photoactive (open during the day and close at night). But in succulents like Bryophyllum, Opuntia and Cacti, stomata close during the day and open at night (scotoactive).
16.Trarispiration in old stems and fruits occurs through lenticels.
17.Fresh weight of a plant or leaf would be maximum in the morning and minimum in the afternoon.
18.If half of the total number of stomata on a leaf close down, the rate of transpiration is not reduced by half.
19.Cytokinins helps in opening of stomata while ABA (abscisic Acid) and low O2 close stomata.
20. Plants growing at high altitudes show xeromorphy (adaptation to minimise transpiration).
21. Transpiration ratio: the amount of water lost per unit of dry matter produced during the growing season of a plant.
22.Plants growing at high altitudes show xeromorphy (adaptation to minimise transpiration)
21.Transpiration ratio: the amount of water lost per unit of dry matter produced during the growing season of a plant.
22.In Saxifraga, the rate of guttation is high during flowering.
23. Mechanical shock causes stomatal closure.
24.Stomatal index = ![]()
25.Transpiration index = ![]()
26.Psychrometer is used to measure relative humidity and rate of transpiration.
27.Diameter of tree decreases during the day. It is due to narrowing of tracheary elements due to development of negative pressure. It is measured by dendrograph.
28.Matric potential Ym: It is used for surfaces which bind water. It is also negative, e.g. soil particles, cell wall, cytoplasm etc.
29.Gravity potential Yg : It denotes the effect of gravity on Yw. It depends on the height (h) of water above the reference state of water, the density at water and acceleration due to gravity. Value of Yg is negligible upto a height of 5m from the reference level and also value of Ym is ignored.
Ψw=Ψs+Ψp
methods to study the mineral requirements of plants
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Methods to study the mineral requirements of plants
Soils normally contain sufficient quantities of essential minerals.
However, three important elements need to be replenished in crop fields as they are depleted by repeated cultivation.
These fertiliser elements called critical elements are nitrogen, phosphorus and potassium (NPK).
The common sources of these elements used in India are: nitrate of sodium, ammonium sulphate, ammonium nitrate, ammonium chloride, urea, etc.
The NPK fetilisers comprising bags of fertilisers are labelled 17-18-19 or 15-15-15 or other combinations.
These numbers refer to the percentage by weight of nitrogen, phosphorus and water soluble potassium.
To determine the elements essential for plant growth and deficiency symptoms of an essential element, well defined nutrient medium has to be used.
Seeds are grown in highly washed pure sand in a glass or glazed procelain or plastic container and supplied with a carefully made up nutrient solution.
Arnon and Hoagland's Medium prescribed a medium containing micronutrients.
Iron was earlier supplied as ferrous sulphate, but it often precipitated out.
This problem has now been solved by dissolving the ferrous sulphate along with a chelating agent Na-EDTA (disodium salt of ethylene diaminetetra acetic acid.)
Solution Culture
It is performed in glass jars or polythene bottles.
The container is covered with black paper after pouring solution into them.
Black paper has two functions -(a) Prevention of growth of algae (b) Prevention of reaction of roots with light.
Seeds are allowed to germinate over split cork.
Cotyledons are removed after seedling formation.
The plant is properly supported with the help of split cork.
Solution is aerated at regular intervals and is changed after 2-3 days.
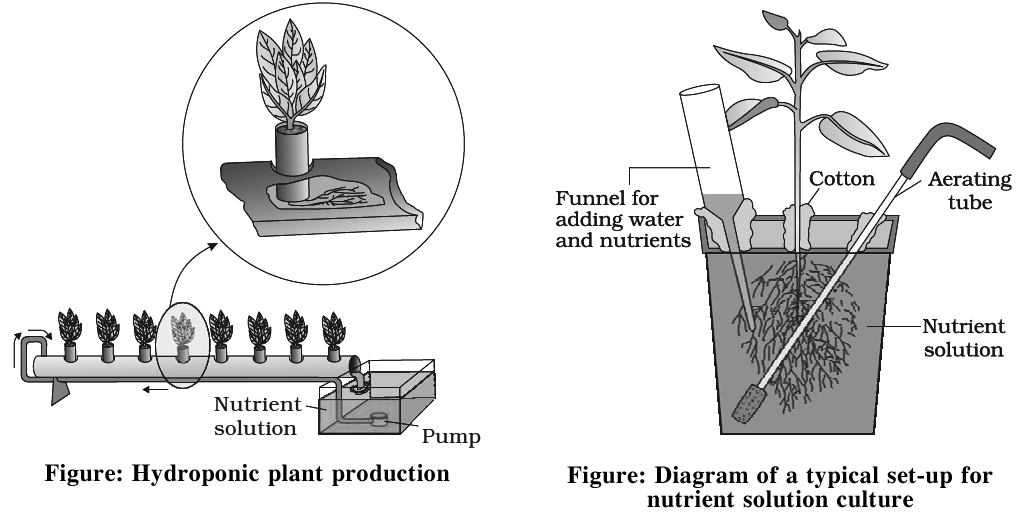
Hydroponics
Commercial technique of soil less culture is called Hydroponics, which was first developed by Goerick (1940).
In 1860, Julius von Sachs, a German botanist, demons rated for the first time, that plants could be grown to maturity in a defined nutrient solution in complete absence of soil.
Culture is performed in large tanks of metal or Reinforced Cement Concrete (R.C.C.) Tanks are covered with wire mesh.
Tanks are provided with aerating and circulating techniques.
Seeds are suspended in solution from the wire mesh with the help of threads.
As plant grows up additional support is provided.
Significance
(i).Useful in areas having thin, infertile and dry soils.
(ii).It can regulate the pH at optimum for a particular crop.
(iii).It controls soil borne pathogens.
(iv).It avoids problem of weeding.
(v).Out of season vegetables (like tomato, seedless cucumber, lettuce) and flowers can also be obtained.
Aeroponics
It is technique of soil-less culture in which roots of plants are suspended in mist of oxygenated nutrient solution.
Sand Culture
In this method, sand is used as a rooting medium and nutrient solution is added to it. It is better than solution cultures w.r.t. providing solid medium and natural aeration for plant. growth. However, this method has following drawbacks:
(i).The sand being highly alkaline in nature, has to be treated with acid before use.
(ii).The sand get very warm during summer and very cool during winters, hence may cause injury to the root system.
(iii).The water holding capacity of sand is very low, hence, it requires freqent watering.
mineral elements that are essential
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Mineral elements that are essential
The inorganic nutrients are classified as essential elements and non essential elements.
17 elements have been placed under essential elements.
These are the elements without which the reproduction and life cycle of a plant cannot be completed.
The essential elements are : C, H, O, N, P, K, S, Mg, Ca, Fe, Mo, Mn, Ni, Zn, B, Cl, Cu.
Essentiality of Minerals
A variety of mineral elements are present in the soil but all of them are not essential for plant growth.
Besides, a particular element may be needed for the growth of one plant and may not be required at all by other plants.
For example, sodium is required in very small amount by the desert shrub Atriplex, but is not required by most of the other plants.
Following criteria given by D.I. Arnon and P.R.Stout (1939) are used to determine essentiality of minerals:
1.The element must be absolutely necessary for normal growth and reproduction. The plant do not complete its life cycle or set the seed in the absence of that particular element
2.The element must not be replaceable by another element.
3.The element must play a direct role in the metabolism of plant.
4.Absence of a specific element causes deficiency in the plant which is corrected only by adding the specific mineral in the soil.
Types of Essential Elements
On the basis of concentration in plant, Hoagland divided essential elements into two groups.
(i) Macronutrients : These are present in more concentration like 1-10 mg per gram of dry weight. These are easily detectable. e.g., C, H, O, N, P, S, Ca, K, Mg.
(ii) Micronutrients : These elements occur in plant body in concentration of equal or less than 0.1 mg per gram of dry weight. Infact these are required in traces, so called trace elements. e.g., Mo, Mn, Zn, B, Cu, Cl, Fe, Ni.
In addition to the 17 essential elements, there are some beneficial elements such as sodium silicon, cobalt and selenium. They are required by higher plants.
General Functions of Mineral Elements
(a) Frame work elements – Form carbohydrates which form cell wall, e.g., C, H, O.
(b) Protoplasmic elements – Form protoplasm, e.g., C, H, O, N, P, S.
(c) Catalytic elements – e.g., Fe, Cu, Zn, Mo, Mg, Mn, K (activator of over 40 enzymes)
(d) Balancing element – Ca, Mg and K counteract the toxic effect of other minerals.
(e) Storage elements – C, N, S, P.
(f) Critical elements – N, P, K.
(g) Minerals influence OP and TP.
(h) Monovalent cations (Na+, K+) Increases permeability of membrane, while divalent and trivalent ions decrease it.
(i) Toxic elments e.g., Al, As, Hg, Pb, Ag.
(j) Non mineral elements e.g., C, H, O, N. N is both mineral and non mineral.
(k) Functional elements: They are non essential in most plants but have a definite activity in some species e.g., silicon in grasses.
absorption of elements
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Absorption of elements
Minerals are mainly absorb by the root which is in direct contact with the soil solution.
Maximum mineral absorption occurs through zone of cell elongation.
The process of absorption can be demarcated into two main phases.
In the first phase, an initial rapid uptake of ions into the 'free space' or 'outer space' of cell -the apoplast, is passive.
In the second phase of uptake, the ions are taken in slowly into the 'inner space' –the symplast of the cells.
The symplast movement requires metabolic energy, i.e., it is an active process.
The movement of dissolved substances into and out of cell is called transport or flux.
Many theories have been given to explain the mechanism of mineral salt absorption.
These theories can be grouped into following two categories:
(1) Passive mineral absorption
(2) Active mineral absorption
(1) Passive absorption
Absorption of ions without use of metabolic energy is known as passive absorption.
Molecules or ions diffuse from a region bf their higher concentration to a region of their lower concentration.
The movement of mineral ions into root cells as a result of diffusion is called passive absorption.
Main theories for passive absorption are described below:
(a) Ion exchange: This theory was proposed by Jenny and Overstreet (1938). Exchange of anions and cations absorbed on colloidal fraction of the soil (clay and humus) with the ions adsorbed on root surface is referred to as ion exchange.
(i) Contact Exchange : This is based on the ion exchange from one adsorbent to another without the participation of free electrolyte. An ion which is adsorbed electrostatically to a solid particle is not tightly bound, but oscillate within a small volume of space. This is termed oscillation volume. According to this concept, H+ ions exchange with the cations and OH– ions exchange with anions.
(ii) Carbonic acid exchange: CO2 is released by root respiration, which forms carbonic acid when dissolved in soil water. This carbonic acid dissociate into H+ and HCO3– ions. Released H+ ions exchange with cations and HCO3– ions exchange with anions.
(b) Donnan equilibrium: This mechanism was proposed by Donnan (1911). Entry of ions into the cell across the plasma membrane to maintain electrical equilibrium is called Donnan equilibrium. Some anions or cations get firmly attached to the inner surface of plasma membrane (fixed and non-diffusible ions). To neutralise these, ions of opposite charges gain entrance in the cell passively (against concentration gradient i.e., without energy expenditure).
(c) Mass flow or Bulk Flow Theory. According to Hylmo, the ion absorption increases with increase in transpiration. The ions have been considered to move in mass with flow of water from the soil solution through the root and eventually to the shoot.
Active mineral absorption
The absorption of ions, involving use of metabolic energy is called active absorption. This occurs against the concentration gradient. Energy used in this mechanism comes from metabolic activities, especially respiration.
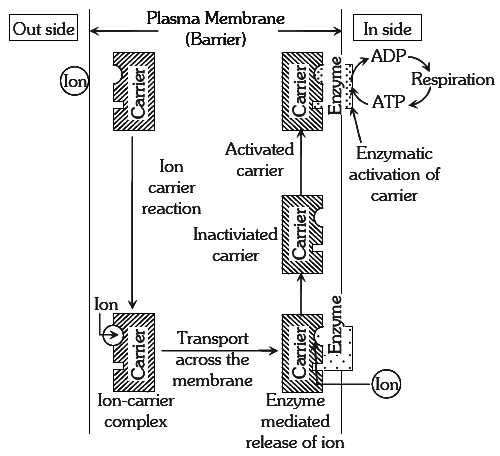
(a) Carrier Concept : This concept was proposed by Van den Honert. According to this concept, there are separate protein carriers for cations and anions. The carrier forms an ion-carrier complex on the outer face of the membrane. This complex dissociates and releases ions into inner-space. The inactivated carrier is again activated by the enzyme kinase. In this process ATP is used up, this activated carrier again accepts new ions and entire cycle is repeated.
(b) Cytochrome Pump Hypothesis - It was proposed by Lundegardh and Burstrom. This gates that anions are absorbed actively and cations passively. At the outer surface of membrane, cytochrome loses an electron durin oxidation and picks up an anion in exchange. It is then transported to the inner side of the membrane through the-cytochrome chain. The cations move passively along the electrical gradient created by the accumulation of anions at the inner surface of membrane. The increased rate of respiration upon anion intake is called as salt respiration.
(c) Protein Lecithin Theory : Proposed by Bennet and Clark. They observed that a phospholipid called lecithin is involved .in transport of ions and act as carrier. The lecithn is composed of phosphatidic acid and choline. Phosphate group in phosphatidic acid is regarded as e active cation binding center and choline is anion binder. These ions are liberated on the inner surface of the membrane by catalysis of lecithin presence of enzyme lecithinase. The regeneration of lecithin from phosphatidic acid and choline occurs in presence of choline acetylase, choline esterase and ATP.
translocation of solutes
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Translocation of solutes
Prerequisites:
Knowledge about methods to study the mineral requirements of plants.
Knowledge of essential elements, their roles and mechanism of absorption of mineral elements in plants.
Concept:
By radio-isotopes, it has been proved that inorganic substances move up the plant through xylem. These substances move along with water by transpiration pull.
The rate at which inorganic solutes are translocated through xylem corresponds to the rate of translocation of water. After absorption of minerals by roots, ions are able to reach xylem by two pathways apoplast and symplast pathway.
soil contains essential elements
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Soil contains essential elements
Prerequisites:
Knowledge about methods to study mineral requirement in plants.
Knowledge of essential elements and their roles in the plant body.
Basic concept about mechanism absorption of elements.
Concept:
Soil provides anchorage, air, water and minerals to the plants growing in it.
Majority of the nutrients that are essential for the growth and development of plants become available to the roots due to weathering and breakdown of rocks. These processes enrich the soil with dissolved ions and inorganic salts. Since they are derived from the rock minerals, their role in plant nutrition is referred to as mineral nutrition.
Soil consists of a wide variety of substances. Soil not only supplies minerals but also harbours nitrogen-fixing bacteria, other microbes.
Since deficiency of essential minerals affect the crop-yield, there is often a need for supplying them through fertilizers.
Both macro-nutrients (N, P, K, S, etc.) and micro-nutrients (Cu, Zn, Fe, Mn, etc.) form components of fertilizers and are applied as per need
Metabolism of Nitrogen
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Nitrogen is the most prevalent element in living organisms.
The atmosphere contain near about 78% of N2 by volume.
Plants compete with microbes for the limited nitrogen that is available in soil.
Thus, it is a limiting nutrient for both natural and agricultural ecosystems.
N2 cycle can be conveniently discussed under the following steps
(i) N2 fixation (ii) Ammonification (iii) Nitrification (iv) Denitrificatio
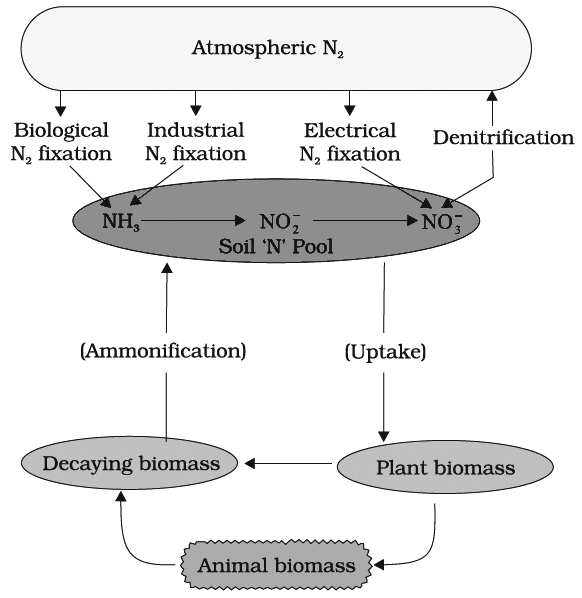
(I) NITROGEN FIXATION
Nitrogen exists as two nitrogen atoms joined by a very strong triple covalent bond (N º N).
The process of conversion dinitrogen (N2) to ammonia is termed as nitrogen fixation.
Following are the methods of N2-fixation:
A. Physico-Chemical method : During thunder, lightening and by using UV rays, atmospheric N2 and oxygen combine to form oxides of nitrogen which form nitrous and nitric acid with water. This may form nitrates of calcium, potassium and ammonium.
B. Industrial N2 fixation: Industrial combustions, forest fires, automobile exhausts and power generating stations are also sources of atmospheric nitrogen oxides.
C. Biological N2 fixation : Only certain prokaryotic species are capable of fixing N2. Biological N2 fixation may be asymbiotic, symbiotic or through loose symbiosis. Biological N2 fixation is called diazotrophy and agents of this process are called diazotrophs.
Some important N2 fixing organisms
(a) Asymbiotic N2 fixers:
Bacteria
(i) Aerobic – Azotobacter, Beijerinckia
(ii) Facultative Aerobic – Klebsiella, Bacillus
(iii) Anaerobic – Clostridium
(iv) Photosynthetic – Chromatium, Rhodospirillum
Blue Green Algae – Anabaena, Aulosira, Nostoc, Scytonema etc. Heterocyst is present in these blue green algae which is responsible for N2 fixation
(b) Symbiotic N2 fixers:
(i) In root nodule of legumes – Rhizobium
(ii) In root nodule of Alnus, Casuarina, Myrica – Frankia
(iii) In leaf nodule of Dioscorea, Pavetta and Psychotria – Klebsiella
(iv) In coralloid root of Cycas – Anabaena cycadae
(v) In fronds of Azolla – Anabaena azollae
(vi) In thallus of Anthoceros – Nostoc
(c) Intermediate: Loose symbiosis with the roots of Sorghum, Zea etc. by Azospirillum.
Rhizobium -Legume Symbiosis
Principal stages of nodule formation are summarised as follows:
1. Rhizobia are Gram negative aerobic rod-shaped bacteria. This genus is responsible for symbiotic N2 fixation in legumes
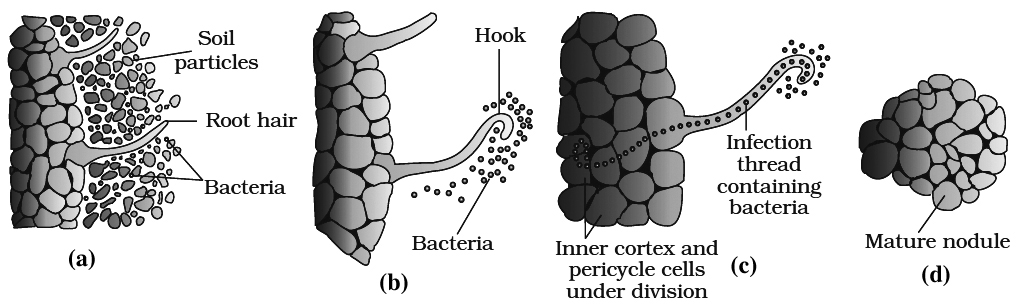
2.Legume roots secrete some specific chemicals (e.g., Flavinoids) which attract the bacteria. Rhizobia multiply an colonise-the surroundings of roots and get attached to epidermal root hair cells.
3.The root hairs curl by the action of nod factors secreted by bacteria and the bacteria invade the root hair.
4.An infection thread is produced, carrying the bacteria into the cortex region of root.
5.Cortical cells are stimulated divide rapidly. It is due to auxins secreted by plants and cytokinins secreted by bacteria.
6.Bacteria enters only polyploid cells of cortex. Some of them enlarge and become membrane bound structures called bacteroids. These form the seat of N2 fixation. These specialised cortical cells now form nodules. Nodules establish a direct vascular connection with the host for exchange of nutrients.
7.The nodules contain a red coloured pigment called leghaemoglobin (LHb). The globin part of leghaemoglobin is formed by host genome, while the heme portion is formed by bacteria.
8.This pigment is O2 carrier and is also called scavenger of O2.
9. Nitrogenase enzyme (synthesized by nif genes of bacteria) is required to fix N2. It is an O2 sensitive enzyme made up of two unequal sub units. Large component has Fe-Mo moiety, while, small component has only Fe-moiety. Here Mo acts as an acceptor and donor of electrons, when N2 is reduced to NH3 LHb maintains anaerobic conditions.
10.N2 fixation requires energy, so it is an active process.
N2 + 8e– + 8H+ + l6 ATP —® 2NH3 + H2 + 16ADP + 16Pi
11.N2 fixation occurs under the controJ of plant nod gene and bacterial nod, nif and fix gene cluster.
12.During this process, atmospheric N2 is reduced by the addition of hydrogen atom.
13.Strong reducing agents e.g., NADPH2, FMNH2, Ferredoxin are also required.
14.Donor of electron and H+ is generally glucose-6-phosphate; certain cofactors like -TPP, Mg++ and CoA are also involved.
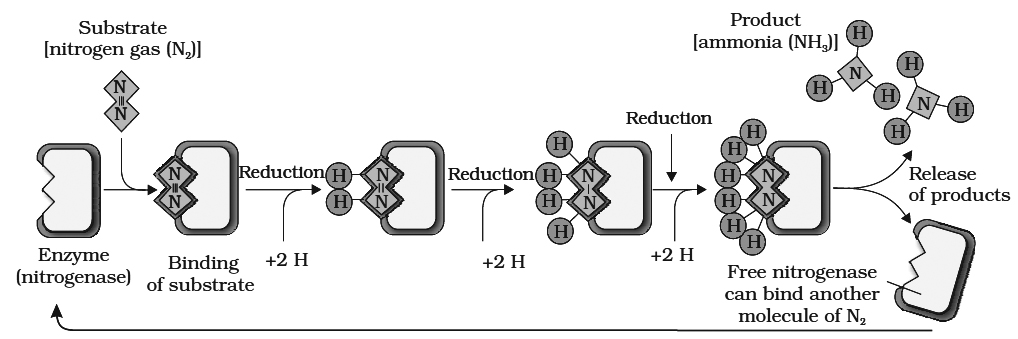
15. ATP is provided by the host respiration process.
16.NH3 so formed is used for the synthesis of amino acids. These acts as building blocks for the synthesis of various types of protein.
(II) AMMONIFICATION
Plants absorb inorganic nitrogen and convert it into proteins
After the death of organisms and plants, proteins are broken into ammonia by the following two steps:
(a) Proteolysis: It is breakdown of protein
![]()
(b) Deamination : Ammonia is released from the amino acids.
Amino acid + H2O ![]() organic acid + ammonia
organic acid + ammonia
It is done by Bacillus ramosus, B. vulgaris, and B. mycoides. This ammonia is converted into nitrate which is absorbed by' the plants.
(III) NITRIFICATION
It is oxidation of ammonia into nitrate, it involves following steps:
(a) Conversion of ammonia into nitrate
2NH3 + 3O2![]() 2NO2– + 2H+ + 2H2O + Energy
2NO2– + 2H+ + 2H2O + Energy
(b) Conversion of nitrite into nitrate.
2NO2– + O2 ![]() 2NO3– + energy
2NO3– + energy
Nitrate assimilation : Nitrate cannot be used by the plant as such. It is first converted into ammonia before being incorporated into organic compounds. Nitrate is reduced in two steps -
The process of nitrate reduction to ammonia is called nitrate assimilation and is accomplished in two steps mediated by two specific enzymes:
(a) First, the nitrate is reduced to nitrite by an enzyme called nitrate reductase. This enzyme is a flavoprotein and contains molybdenum.
(b) The nitrite ions are then reduced to ammonia by an enzyme called nitrite reductase. Ferredoxin is the most direct source of electrons for nitrite reduction and hence, it occurs specifically in leaves. Therefore, nitrite ions formed in other parts of the plant are tranported to leaves and further reduced to ammonia. Nitrite reductase does not require molybdenum but contains copper and iron.
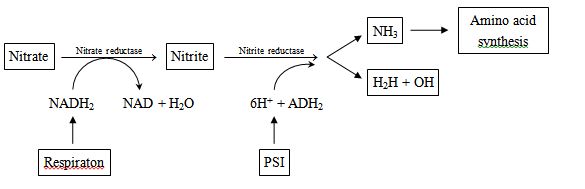
Ammonia thus formed, is fixed by the organic acids to produce amino acids which form other nitrogenous compounds.
(IV) DENITRIFICATION
Nitrates are broken down into gaseous nitrogen or nitrolls oxides by some microorganisms.
It is called denitrification e.g., Thiobacillus denitrificans, Micrococcus denitrificans, Pseudomonas denitrificans.
FATE OF AMMONIA
At physiological pH, the ammonia is protonated to form NH4+ ions, which is quite toxic to plants and hence cannot be accumulated by them. This is commonly used up to synthesize
(a) Amino acids and or (b) Amides
(a) Amino Acids
Amino acids are generally the initial products of N2 assimilation. Mainly, these two processes are used by plants to synthesise amino acid :
(i) Reductive Amination : In this process, NH3 reacts with a-ketoglutaric acid and forms glutamic acid.
a-Ketoglutaric acid + NH4+ + NAD(P)H Glutamic acid + H2O + NAD (P).
(ii) Transamination : It involves the transfer of amino group from one amino acid to the keto group of other keto acid.
Glutamic acid is the main amino acid from which other 17 amino acids are formed through transamination. Enzyme required for this reaction is transaminase.
(b) Amides
Two most important amides present in plants are asparagine and glutamine.
These are formed from two amino acids, glutamic acid and aspartic acid.
For this reaction, glutamine synthetase and asparagine synthetase are required respectively.
Amides contain more N2 than amino acids and are structural part of most proteins, these are transported through xylem vessels.
Inaddition, along with the transpiration stream the nodules of some plants (e.g., soyabean) export the fixed nitrogen as ureides (allantoin, allantoic acid and citrulline).
Important definitions/ formulae:
Some important N2 fixing organisms
(a) Asymbiotic N2 fixers:
Bacteria
(i) Aerobic – Azotobacter, Beijerinckia
(ii) Facultative Aerobic – Klebsiella, Bacillus
(iii) Anaerobic – Clostridium
(iv) Photosynthetic – Chromatium, Rhodospirillum
Blue Green Algae – Anabaena, Aulosira, Nostoc, Scytonema etc. Heterocyst is present in these blue green algae which is responsible for N2 fixation.
(b) Symbiotic N2 fixers:
(i) In root nodule of legumes – Rhizobium
(ii) In root nodule of Alnus, Casuarina, Myrica – Frankia
(iii) In leaf nodule of Dioscorea, Pavetta and Psychotria – Klebsiella
(iv) In coralloid root of Cycas – Anabaena cycadae
(v) In fronds of Azolla – Anabaena azollae
(vi) In thallus of Anthoceros – Nostoc
(c) Intermediate: Loose symbiosis with the roots of Sorghum, Zea etc. by Azospirillum.
Prior knowledge of the photosynthesis and early experiments
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Prior knowledge of the photosynthesis and early experiments
Some important events related to photosynthetic process are described below.
(1) Stephan Hales was the first to prove that gaseous constituents of the air and light also contribute to the building up of the plant body.
(2) Joseph Priestley concluded that plants purify the impure air released by animals. Joseph Priestley (1733-1804) in 1770 performed a series of experiments that revealed the essential role of air in the growth of green plants. Priestley discovered oxygen in 1774.
Priestley observed that a candle burning in a closed space -a bell jar, soon gets extinguished (Figure a, b, c, d). Similarly, a mouse would soon suffocate in a closed space. He concluded that a burning candle or an animal that breathe the air, both somehow, damage the air. But when he placed a mint plant in the small bell jar, he found that the mouse stayed alive and the candle continued to burn. Priestley hypothesised as follows: Plants restore to the air whatever breathing animals and burnning candles remove.
(3) Jan Ingenhousz mentioned, that in sunlight green leaves give out dephlogisticated air (air rich in oxygen) and in dark these green parts give out phlogisticated air (air rich in CO2) and make the air impure.
(4) Jean Senebier discovered that green plants utilize carbon dioxide. It was thus a great land mark in the discovery of photosynthesis.
(5) N.T. de Saussure proved that the volume of carbon dioxide consumed is equal to the volume of oxygen liberated. He also showed that water is essential requirement in photosynthesis.
(6) Pelletier and Caventou named the green coloured substance in the leaves as chlorophyll.
(7) F.F. Blackman observed that the process of photosynthesis has two steps : a photochemical reaction which proceeds only in the presence of light and a dark reaction for which light is not necessary. Besides this, he also proposed the law of limiting factor.
(8) Warburg performed Flashing experiment on Chlorella.
(9) Emerson and Arnold, with the help of experiments determined that the light reaction of photosynthesis has two distinct photochemical processes.
(10) Robert Hill's experiment on Stellaria media demonstrated that in the presence of sunlight, water and a suitable hydrogen acceptor, isolated chloroplasts release oxygen, even if carbon dioxide is absent. This experiment is considered to be equivalent to light reaction. Hill's oxidants are hydrogen acceptors. The common ones are ferricyanide, benzoquinone and dichlorophenol indophenol(DCPIP), while NADP+ is natural H+ acceptor in photosynthesis.
(11) Van Niel, based on his studies of purple and green sulphur bacteria demonstrated that hydrogen released from a suitable oxidisable compounds reduces CO2 to carbohydrates and proposed that water is the source of O2.
(12) Ruben, Hassid and Kamen using radioactive oxygen (O18) confirmed that the source of oxygen in photosynthesis is water.
(13) Julius von Sachs (1854) found that the green parts in plants is where glucose is made and glucose is usually stored as starch.
(14) T.W. Engelmann experimented on Cladophora and Spirogyra. He used a prism to split light into its components and illuminated the green alga placed in a suspension of aerobic bacteria. He observed the accumulation of bacteria in the region of blue and red light of the split spectrum. Thus, he described the first action spectrum of photosynthesis.
(15) Melvin Calvin and his coworkers discovered various reactions involved in the conversion of carbon dioxide into carbohydrates using C14O2. These reactions are collectively known as C3 cycle or Calvin cycle. He was awarded Nobel Prize for this work in 1961.
(16) M.D. Hatch and C.R. Slack discovered a new series of reactions involved in photosynthetic CO2 assimilation in many tropical plants. In these plants the first stable compound in photosynthetic process is a 4-carbon compound, hence, this series of reactions is known as C4 cycle.
(17) Hill and Bendall proposed the scheme of light reaction.
(18) Huber et. al. studied three dimensional structure of reaction center of bacteria Rhodopseudomonas viridis and got Nobel prize.
where does photosynthesis occur?
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Photosynthesis occur
In higher plants chloroplast is semiautonomous, endosymbiont with three membrane system. It is made up of three main parts: Envelope, Matrix and Thylakoid.
Envelope: It is made up of outer membrane ±porins, inner membrane, in between outer and inner membrane is the periplastidal space.
Semi fluid matrix known as stroma, which contain double stranded circular DNA, 70S ribosome,
Carboxylation enzyme RUBISCO, and PEP carboxylase enzyme Mg, Cl, Mn etc.
Thylakoid is membrane lined flattened sac like structure unite to form Grana.
Each thylakoid in grana is known as grana thylakoid, inside it contains the lumen for photolysis of water with the help of Mg, Mn, Cl, Ca and number of electron carriers.
Each grana interconnected with stroma lamellae. Inside it contains space known as fret channel.
Thylakoid membrane possesses 200 to 400 pigment molecule associated with certain protein known as CAB together known as quantosomes or light harvesting complex.
Thylakoid membrane also possess F0 -F1 particle or coupling factor and number of electron carriers arranged based on their redox potential
Photosynthetic Pigments
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Photosynthetic Pigments
There are mainly three types of pigments: chlorophylls, carotenoids and phycobilins (in BGA and red algae).
(1) Chlorophylls (green pigments)
The chlorophylls are main pigments which plants use in Photosynthesis.
They are specialized lipid molecules embedded in thylakoid membrane.
They are the main pigment concerned with harvesting or trapping of solar energy. Arnof and Allen (1966) recognised nine types of chlorophyll – (i) chlorophyll a; (ii) chlorqphyll b, (iii) chlorophyll c, (iv) chlorophyll d; (v) chlorophyll e; (vi) bacteriochlorophyll a, (vii) bacterio -chlorophyll b, (viii) chlorobium chlorophyll 650 and (ix) chlorobium chlorophyll 666.
Out of these, the two important types of chlorophyll found in green plants are chlorophyll a and
Of these, chlorophyll a is the primary photosynthetic pigment.
It is found in all photosynthetic eukaryotes and blue green algae (cyanobacteria).Light energy absorbed by all other types of chlorophylls and accessory pigments is ultimately transferred to chlorophyll a.
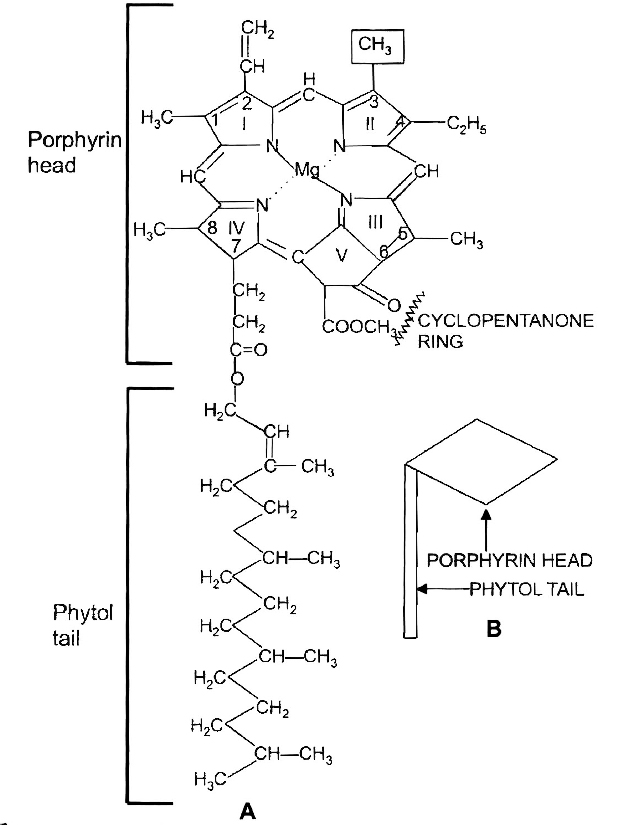
(a) Chlorophyll a : The chlorophyll a has a empirical formula as C55H72O5N4 Mg. The molecule is distinguishable into a 'head' of size 15 × 15 Å and a 'tail' of 20 Å length. The 'head' is made up at porphyrin, (a tetrapyrrole closed ring derivative) and the 'tail' of phytol (C20H39OH).
There is a 5th isocyclic ring of cyclopentanone. A non-ionic magnesium atom is held within tetrapyrrole ring by two covalent and two co-ordinate bonds. There is vinyl group at carbon-2 position and methyl at carbon-3. The chlorophyll a absorbs violet blue and red wavelengths of the spectrum giving peaks at 430 and 662 nm respectively. It is found in all photosynthetic organisms except bacteria. Protoclorophyll is its precursor.
(b) Chlorophyll b : Its empirical formula is C55H70O6N4 Mg. It is similar to chlorophyll a except in having a formyl (CHO) group instead of methyl (CH3) at carbon-3 position of the tetrapyrrole ring. It absorbs blue and orange wavelengths giving peaks at 430 and 644 nm.
(c) Chlorophyll c: It lacks phytol esterification. It is found in brown algae, diatoms and dinoflagellates.
(d) Chlorophyll d: It has formyl group at carbon-2 position. It is reported from red algae.
(e) Chlorophyll e: It has been reported from Xanthophyceae members like Vaucheria.
(f) Bacteriochlorophyll: The molecular formula of bacteriochlorophyll-a is C55H74O6N4 Mg. Its structure is similar to chlorophyll a except presence of acetyl group instead of vinyl at carbon-2 position.
(g) Chlorobium chlorophyll: It has hydroxy-methyl group (CH3CHOH) at carbon-2 position in the tetrapyrrole nucleus.
(2) Carotenoid pigments (Accessory or shield pigments):
Carotenoids are yellow to orange lipid compounds which occur in almost all higher plants. Carotenoids are of two types-carotenes and xanthophylls.
(a) Carotenes are reduced molecules (C40 H56). They are of several types like a and b.
b-carotene is the most widespread and important carotene associated with chlorophyll in photosynthetic organisms. It is orange-yellow in colour. Vertebrates are able to break each molecule of b-carotene in two molecules of vitamin A during digestion. Red colour of tomatoes is due to a carotene called lycopene.
(b) Xanthophylls. These pigments, besides carbon and hydrogen also contain oxygen, e.g., lutein (C40H56O2), cryptoxanthin (C40H56O), etc.
Carotenoids have at least two major roles in photosynthesis: (i) they transfer the light energy absorbed by them to chlorophyll molecules, hence are called antenna molecules. They absorb light in blue violet range and (ii) they protect plant from excessive heat and prevents photooxidation (oxidative destruction by light) of chlorophyll.
(3) Phycobilins (Biliprotein):
Phycobilins are another group of accessory pigments found in red and blue-green algae. They have open chain tetrapyrrole structure and are hot water-soluble pigments. Phycobillins can be classified into two types: (i) Phycoerythrins, (ii) Phycocyanins.
Usually both these types occur together, but their proportion may vary according to species and environment.
Mechanism of Photosynthesis
The two major steps of photosynthesis are -Light or Hill reaction and dark or Blackman reaction.
Concept Builder
Flashing (Intermittent) light experiment was performed by Warburg. He established that the rate of photosynthesis was found to be greater in intermittent light as compared to continuous light. It indicates the existence of two steps in photosynthesis, a light dependent reaction and another light independent reaction (the dark reaction, enzymatic reaction).
Photosynthetic Units / Pigment Systems
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Photosynthetic Units
These are group of pigment molecules which takes part in change of light energy into chemical energy.
Each photosynthetic unit has a reaction center (photocentre) of specific chlorophyll a molecule like P700 and P680, which absorb light energy of long wavelength.
These can release electron upon absorption of energy.
Reaction center is surrounded by a number of light harvesting pigment molecules.
These are those photosynthetic pigments which absorb photons of different wavelength.
Harvesting molecules occur in the form of specific complexes called LHC I and LHC II.
Harvesting molecules are of two types :
I. Antenna molecule : Those occurs on outer side of photosynthetic unit. They absorb photons of different wavelength depending upon their absorbance maxima. They get excited and energy is transferred to core molecule through resonance transfer.
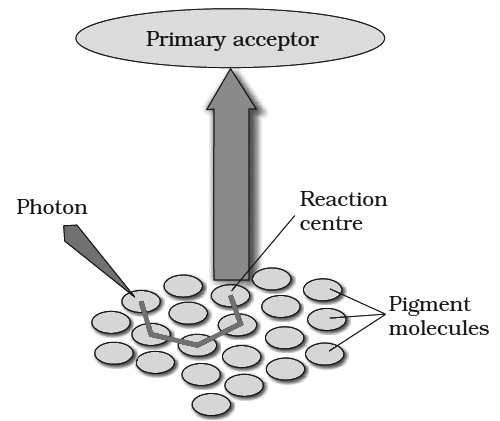
II. Core molecules: They are pigment molecules which are present around the reaction center. They transfer the energy to reaction center, so the reaction center gets excited.
(3) Evolution of oxygen :
Equations of photosynthesis proposed before 1930s gave the impression that the source of oxygen was carbon dioxide.
However, experiments of C.B. Van Niel performed during 1930s showed that oxygen gas liberated during photosynthesis is released as a result of photolysis of water.
While comparing photosynthetic activity of sulphur bacteria and higher plants, Van Niel found that sulphur bacteria assimilate carbon dioxide using hydrogen sulphide (H2S) as a hydrogen source, whereas in higher plants the source of hydrogen is water. 6CO2 +12H2S ![]() C6H12O6 + 6H2O + 12S ... (1)
C6H12O6 + 6H2O + 12S ... (1)
6CO2 +12H2O![]() C6H12O6 + 6H2O + 6O2 ... (2)
C6H12O6 + 6H2O + 6O2 ... (2)
In both the above processes carbon dioxide is used but no oxygen is evolved by sulphur bacteria, while on the other hand, it is evolved in higher plants.
It confirms that source of oxygen is water and not carbon dioxide as was believed earlier.
On this basis Van Niel gave following generalized equation for photosynthesis in higher plants and bacteria:
CO2 + 2H2A ![]() (CH2O) + H2O + 2A
(CH2O) + H2O + 2A
In this presentation, H2A represents a hydrogen donor; in green plants this is water (H2O) and in sulphur bacteria it is hydrogen sulphide (H2S).
The action of light might be to produce a reducing entity [H] and an oxidising entity [O] from water.
The views of Van Niel were further confirmed by Ruben, Hassid and Kamen (1941) using radioactive isotope of oxygen (O18).
When they used water (containing radioactive oxygen, H2O18), (O18) was evolved.
It thus confirms Van Niel's observation that the source of oxygen evolved in photosynthesis is water.
Equation (2) provides the most adequate explanation of photosynthetic process, since it shows that oxygen evolved during photosynthesis comes from water.
(4) Red Drop:
Robert Emerson measured the efficiency of photosynthesis of Chlorella cells in monochromatic light (i.e. only one wavelength of light at a time).
They observed that the quantum yield of photosynthesis decreased very sharply towards 680nm and longer wavelengths.
Since this decrease in quantum yield is observed at the far-red region of the spectrum, hence it is called red drop.
Quantum requirement: It is the number of light quanta needed for production of one O2 molecule or reduction of one CO2 molecule. It is 8.
Quantum yield: It is the number of oxygen molecules evolved per light quanta absorbed. Two light quanta are required to move one electron. Such four electrons are involved in the evolution of one molecule of oxygen. Eight quanta are, therefore required for evolution of one oxygen molecule. Thus quantum yields or number of oxygen molecules evolved per light quanta is 1/8.
Enhancement Effect
Emerson found that when light of shorter wavelengths was used superimposed with the light of long wavelengths, the rate of photosynthesis was greater (25% more) than could be expected from adding the rates found when either light was given alone.
Thus both light beams had a synergistic effect i.e. one helped the other.
It leads to the conclusion that two group of pigments, one absorbing in lower range and other absorbing in higher range of wavelengths are involved in photosynthesis and are called pigment systems.
(5) Two pigment systems :
It is a group of pigment molecules having chlorophyll a, chlorophyll b, carotenoids etc.
One molecule of chlorophyll a functions as a reaction centre or trapping centre and others act as light harvesting complex (LHC) or antenna molecules.
These antenna molecules collect light energy and transfer this energy (by inductive resonance process) to reaction centre where primary photochemical act occurs (quantum conversion) i.e. absorption of a photon and release of an electron.
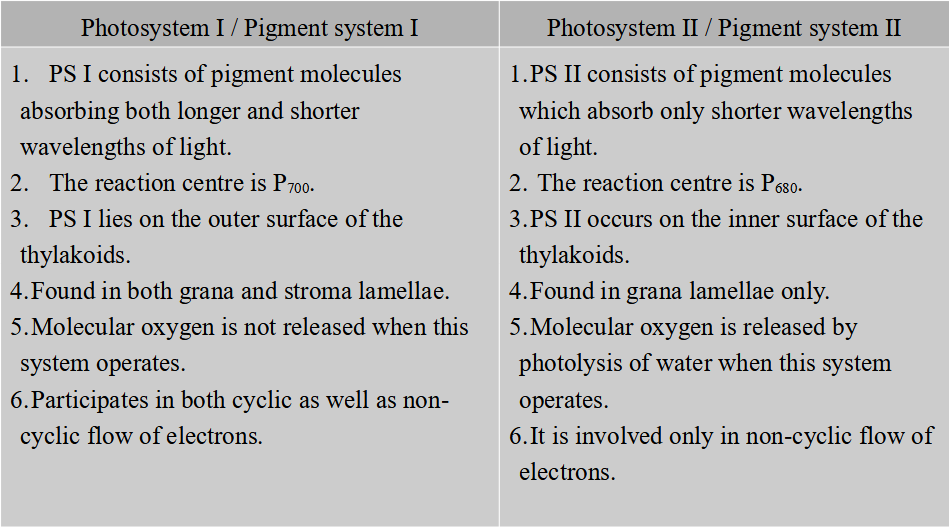
Mechanism of Photosynthesis
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Mechanism of Photosynthesis
LIGHT REACTION OR HILL REACTION
It occurs in lamellar part of chloroplast an antasomes are units of light reaction. It includes following main processes.
(1) Light and its absorption :
White light, as it reaches us from the sun, is composed of different wavelengths.
When passed through a glass prism it resolves into seven colours-violet, indigo, blue, green, yellow, orange and red.
This band of seven colours constitutes the visible spectrum.
Visible light consists of radiations having a wavelength between 390-760 nm.
Part of spectrum used in photosynthesis has a wavelength between 400 -700 nm and is called PAR i.e., photosynthetically active radiation.
In the range of visible spectrum violet bas the shortest wavelength (390-430 nm) and red the longest (660-760nm).
Wavelength longer than 760 nm (infra red radiation) and shorter than 390 nm (ultraviolet radiation) also exist but these are invisible.
Only a small portion of visible spectrum reaction the earth is utilized in photosynthesis.
Chlorophyll - the green pigment of leaves, absorbs mainly the blue 470 -500 nm) and red (660 -760 nm) wavelengths.
It reflects the green wavelengths (500-580 nm) and that is why the leaves appear green in colour.
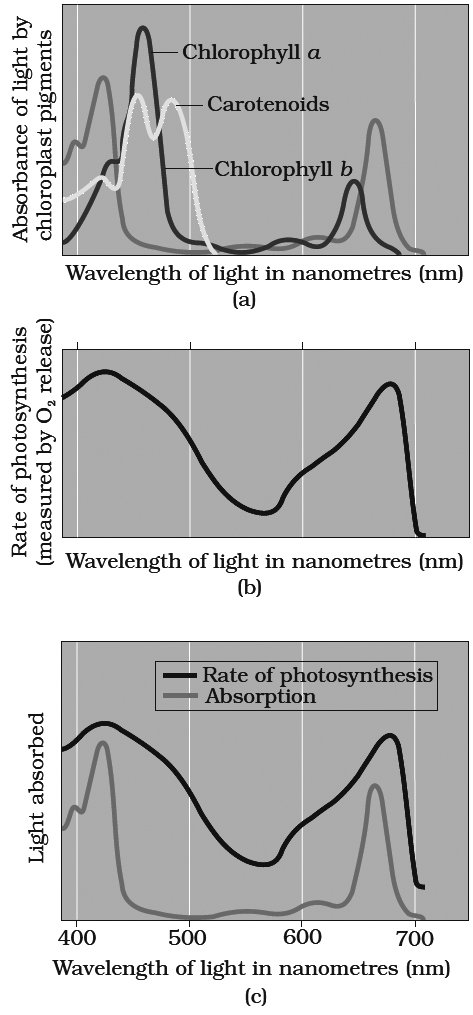
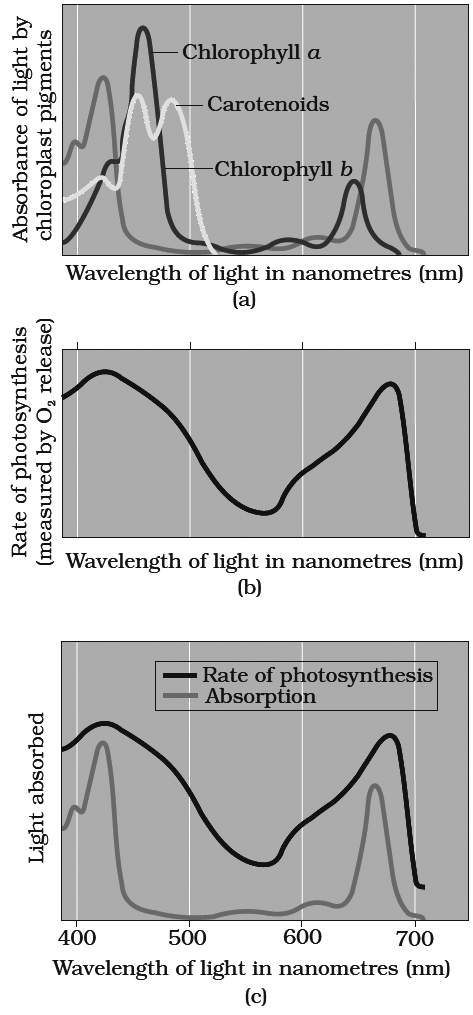
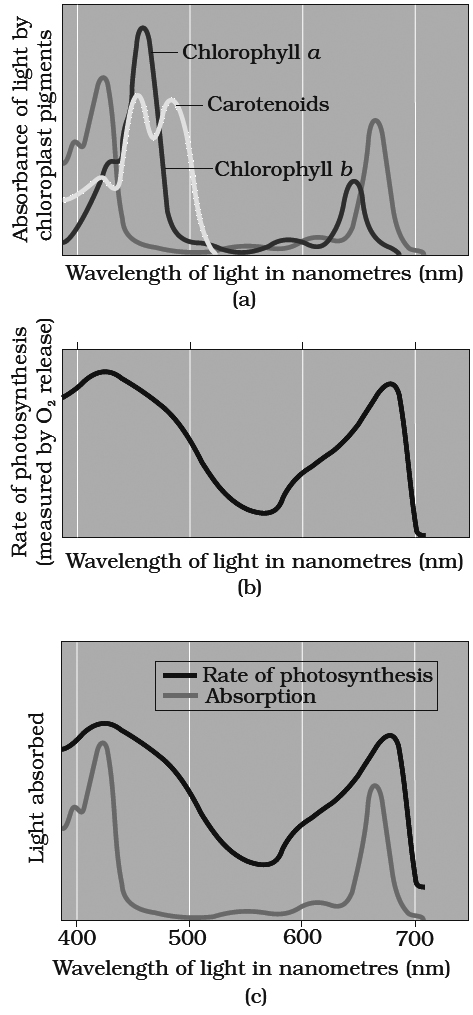
Graph (a) showing absorption spectra of chlorophylls a, b and carotenoids.
Graph (b) showing action spectrum of photosynthesis. Graph (c) showing action spectrum of
photosynthesis superimposed on absorption spectrum of chlorophyll a
Absorption and Action Spectra
Different plant pigments absorb only certain wave lengths of light and these wavelengths are not absorbed at the same rate.
A curve obtained by plotting the amount of absorption of different wavelengths of light by a particular pigment is called absorption spectrum.
An action spectrum is a curve showing the effectiveness of different wavelengths of light in stimulating the process being investigated.
The effectiveness of different wavelengths of light on photosynthesis is measured and plotted by quantum yield or amount of action (expressed through CO2 reduction, O2 evolution etc.) Action spectrum of photosynthetic pigments was studied by Engleman on Cladophora and Spirogyra for the first time.
(2) Excitation of chlorophyll by light :
Each molecule of chlorophyll is composed of many atoms of hydrogen, oxygen, carbon, nitrogen, etc. Negatively charged electrons of different energies revolve in specific orbits around positively charged nucleus of the atom.
Each electron possesses a definite amount of energy depending upon its distance from the nucleus.
This state of electron is known as ground state -a condition of maximum stability.
If a sufficiently energetic photon of light strikes one of the electrons, the electron acquires the energy of photon (quantum) and jumps to another orbit of higher energy.
This is said to be the excited state of the electron.
It is an unstable state and the electron stays in this state for only a very short time (10–5 – 10–9 second).
As the excited electron returns back to its ground state, the trapped energy may be emitted as heat, light or as fluorescence.
In green plants, however, the energy emitted by the excited chlorophyll molecule is trapped and stored in the form of chemical energy (ATP) rather than being lost simply as light or heat.
If the excited electron returns to its ground state in one step, the energy stored in it would be released in one large dose and may not be fully converted into chemical energy.
Therefore, the excited electron, as it returns to its ground state, is passed on to a number of acceptors so that the maximum amount of released energy is converted into chemical energy.
Concept Builder
Photoluminescence :
It is the property of reradiation of absorbed energy. The reradiated energy is usually or longer wavelength. It of two types.
(i) Fluorescence : It is immediate reradiation of fight energy. It stops immediately after the withdrawal of source of illumination. All types of photosynthetic pigments are fluorescent. Most of the fluorescence produced by green parts are due to chlorophyll-a.
Isolated chlorophyll 'a' when dissolved in organic solvents and exposed to light emits red colour immediately. It is called fluorescence; and this quality is called kutusky effect. It is, infact, a manifestation of loss of light energy absorbed in excess. Actually, when the absorbed energy is not utilised for a purpose (phosphorylation), it is emitted out as radiations of longer wavelength. In green plants, of course, this is utilised to synthesise chemical energy (ATP).
(ii) Phosphorescence: The delayed re-radiation of absorbed energy is called phosphorescence.
Non - Cyclic Photophosphorylation
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Non-cyclic photophosphorylation:
It involves photosystem I as well as photosystem II. Both these systems absorb light photons. For convenience, the non-cyclic photophosphorylation is described here in following steps:

(i) When P680 absorbs a photon of red light, it gives out an electron, and thus becomes positively charged. This electron from P680 passes to P700 through a series of electron carriers, like Phaeophytin (Primary acceptor), plastoquinone, cytochrome b6-f complex, plastocyanin.
This movement of electron is downhill in terms of redox potential scale.
In this series, enough energy is released when the electron is transferred from PQ to cytochrome b6-f complex. It is utilized to synthesize ATP.
(ii) Simultaneously, electron in the reaction centre of PS I also get excited when they receive red light of 700 nm and these electrons are transferred to another receptor having high redox potential. These electrons then move downhill again, this time to a molecule of NADP+. This addition of electron and a proton, reduce it to NADPH + H+.
1.The process requires an external electron donor.
2.It is connected with photolysis of water and liberation of oxygen.
3.It is not only connected with ATP synthesis, but also with production of NADPH.
4.Non-cydic photophosphorylation takes place under optimum light, aerobic conditions and in the presence of carbon dioxide.
5.The system is connected with CO2 fixation.
6.It occurs in the granal thylakoids.
7.DCMU inhibits noncyclic photophospho- rylation.
Splitting of water
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Splitting of water
(iii) Photolysis of water and replacement of ejected electron of P680. Breakdown of water molecules under the influence of light is known as photolysis.
The positively charged P680 molecule (after having given an electron) exerts a strong pull on the water molecule, splitting it into H+ and OH– ions. This is called photolysis of water. It requires presence of OEC (oxygen evolving complex) enzyme, Cl– and Mn++ ions as catalysts (Ca+2 ions control their concentrations).
4H2O![]() 4(OH)– + 4H+ ...(i)
4(OH)– + 4H+ ...(i)
4(OH)– ![]() 4e– + 4OH ...(ii)
4e– + 4OH ...(ii)
The OH– donate their electrons to oxidised P680 so that it once again becomes functional.
The (OH)– radical obtained in equation (ii) above eventually forms water and liberates oxygen.
4(OH)![]() 2H2O + O2 ...(iii)
2H2O + O2 ...(iii)
Hydrogen ions (4H+) formed by the photolysis of water {equation (i)}, accumulate within the lumen of thylakoids.
Redox potential is the tendency of an atom or molecule having low redox potential to lose electrons (electron donors), while those with high redox potential to accept electron (gain electrons).
Hence, electrons move from substances having low redox potentials to those having high redox potentials.
Assimilatory power: NADPH + H+ and ATP produced in light reaction constitute assimilatory power, NADPH + H+ produced in light reaction is called as reducing power.
Chemiosmotic Hypothesis
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Chemiosmotic Hypothesis
Generation of ATP in light reaction is called photophosphorylation.
Chemiosmotic hypothesis was first explained by P. Mitchell.
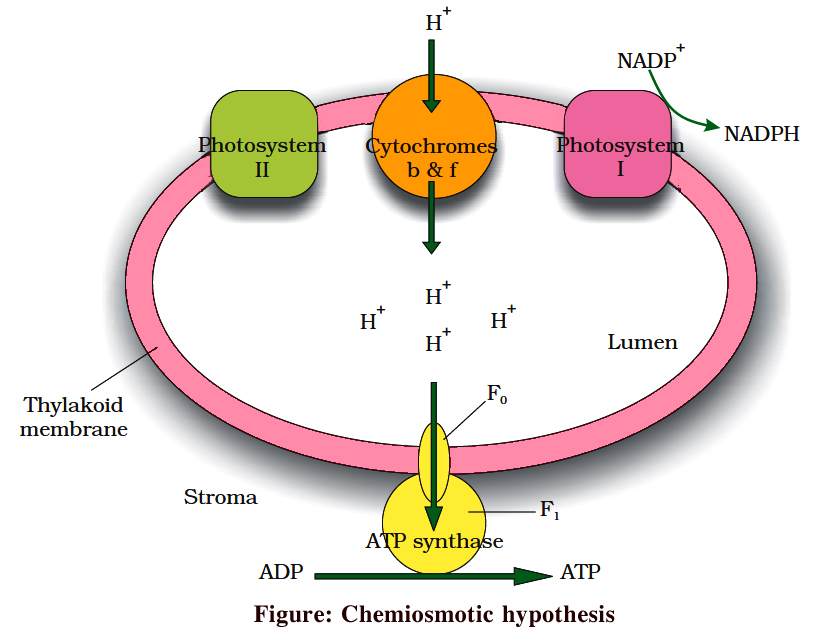
As a result of (a) photolysis towards thylakoid lumen, (b) quinone pump, which takes H+ from stroma to lumen, and (c) NADP+ reductase reaction towards stroma; H+ concentration in thylakoid lumen increases by 1000-2000 times and as a result a proton motive force develops.
This force is responsible for ATP synthesis on the head of CF0 – CF1 particle plugged on thylakoid membrane. The CF0 part forms facilitated channel for proton diffusion towards stroma. Movement of 3H+ is involved in the synthesis of 1 ATP.
Where are ATP and NADPH used ?
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
ATP and NADPH used
DARK REACTION / PCR OR BLACKMAN REACTION
The light independent or dark reaction of photosynthesis reduces the carbon dioxide to glucose.
These reactions take place in the stroma of chloroplast where light independent enzymes are present.
Although this process does not require light, it depends upon the products of light reaction of photosynthesis.
The energy required for the reduction of carbon dioxide is derived from ATP and hydrogen from NADPH (formed in the light reaction of photosynthesis).
The dark reaction occurs through one of the following three methods under different conditions by various plants:
(1) Calvin cycle or C3 cycle
(2) Hatch and Slack cycle
(3) CAM cycle
(1)CALVIN CYCLE or C3 CYCLE
This process comprises a series of reactions controlled by enzymes.
The sequence of these reactions were determined in Chlorella and Scenedesmus by Calvin, Benson and Bassham using radioactive carbon 14C, and techniques like chromatography and autoradiography.
Therefore, it is also known as Calvin cycle or Calvin-Benson cycle.
First product of CO2 fixation is a 3C compound called 3-phosphoglyceric acid (PGA).
This cycle occurs in all photosynthetic plants; whether they have C3 or C4 or CAM pathways. The enzyme for CO2 fixation is RuBisCO.
It is a large enzyme found in stroma.
It is most abundant protein on earth and constitutes 16% of chloroplastic protein.
It shows bifunctional nature having both carboxylase and oxygenase activity.
The dark reaction is also known as Blackman's reaction.
The whole reaction can be studied in three parts:
(i) Carboxylation (Acceptance of CO2 by RuBP i.e., CO2 acceptor -it is a 5C compound),
(ii) Reduction, (iii) Regeneration of RuBP.
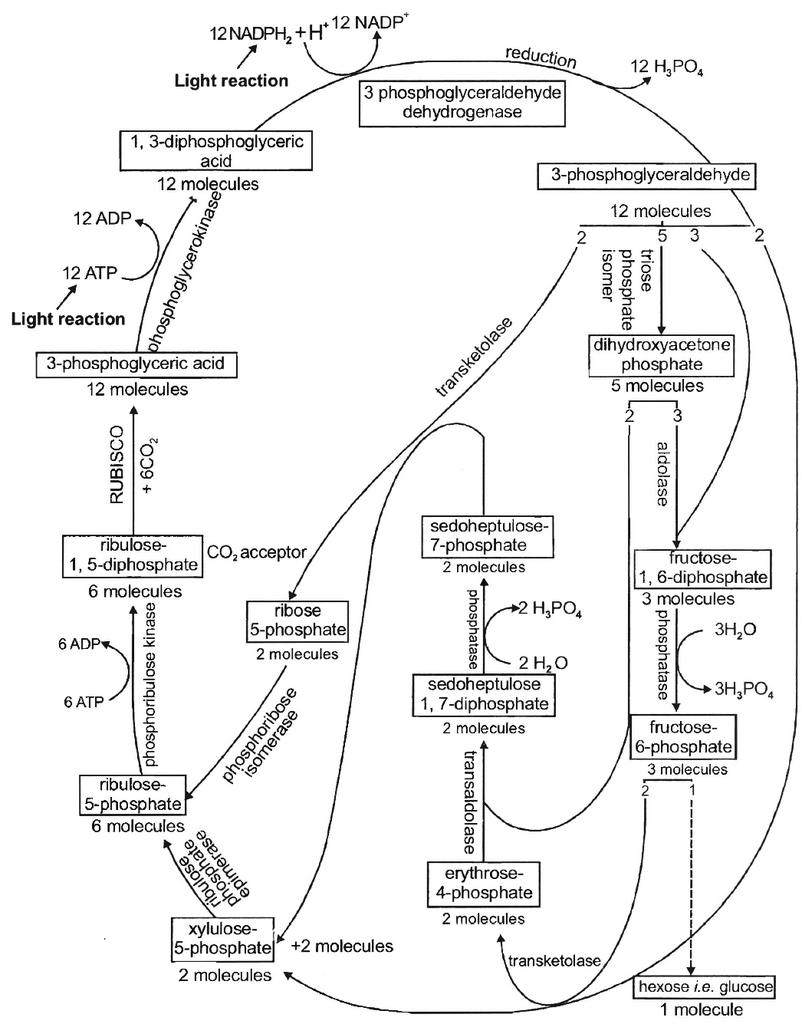
18 ATP and 12 NADPH + H+ are used for the formation of a molecule of glucose (or to reduce 6CO2).
During this process, 264 grams of CO2 and 216 grams of water are used for producing 108 grams of water and 192 grams of O2.
End product of photosynthesis. Hexose sugars are considered as the end products of photosynthesis. Generally, hexose sugar is stored in the form of starch, a long and branched chain of repeating units of simple sugars (usually glucose). Besides, under certain circumstances phosphoglyceraldehyde may also directly or indirectly give rise to fats and proteins in addition to hexose sugars.
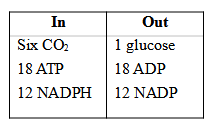
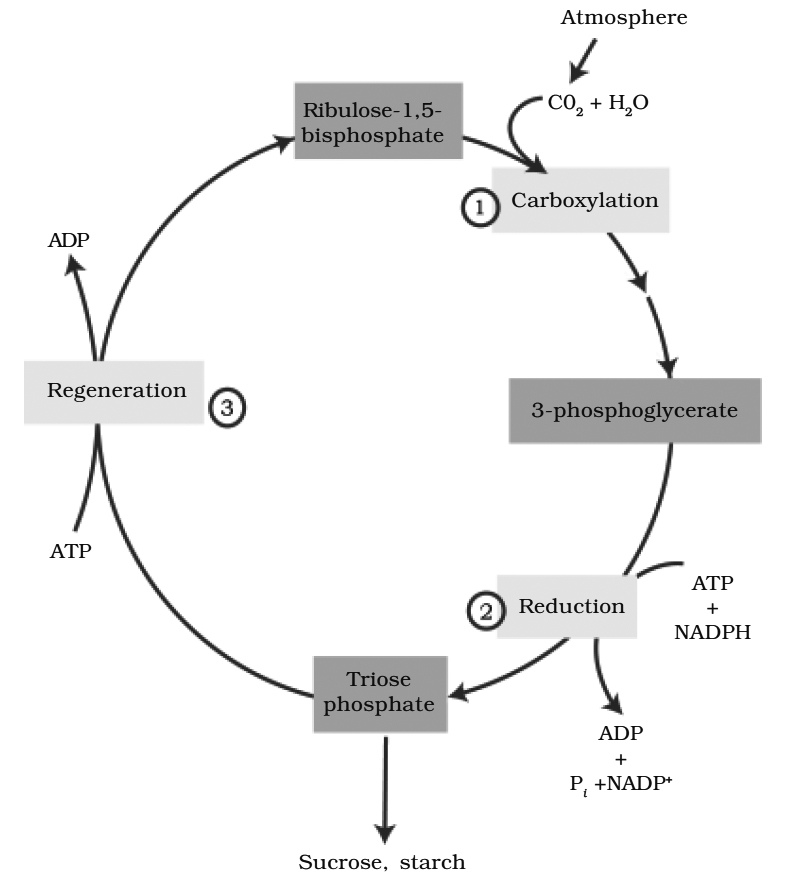
(1) carboxylation, during which CO2 combines with ribulose-1, 5-bisphosphate;
(2) reduction, during which carbohydrate is formed at the expense of the
photochemically made ATP and NADPH; and
(3) regeneration during which the CO2 acceptor ribulose 1, 5-bisphosphate is formed again so that the cycle continues.
The C4 Pathway {Hatch and Slack Pathway}
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
The C4 Pathway {Hatch and Slack Pathway}
OR CO-OPERATIVE PHOTOSYNTHESIS
Most of the plants are known as C3 plants because the first product of CO2 fixation is a 3 C compound, phosphoglyceric acid.
But C3 cycle (or Calvin cycle) is not the only way in plants to fix CO2, H.P. Kortschak and C.E. Hartt (1965) found that in sugarcane (a tropical plant), leaves removed CO2 more efficiently from the atmosphere; and the first products of photosynthesis were acids containing 4-carbon atoms (e.g., malic, oxaloacetic and aspartic acid), rather than the 3C-acid PGA.
Since then, the same has been found true for many important tropical plants including monocots (like maize, Sorghum and Eleusine) as well as dicots (like Amaranthus and Euphorbia sp.).
These plants are called C4 plants. In 1966, two Australian scientists, Hatch and Slack showed that C4 plants are much more efficient in CO2 utilization than C3 plants.
Such plants do not show photorespiration.
In 1967, Hatch and Slack explained the manner of CO2 fixation and reduction in such plants.
The new carbon pathway in C4 plants is called Hatch-Slack Pathway.
The leaves of C4 plants show Kranz anatomy, means there vascular bundles are surrounded by two rings of cells:
(i) Bundle sheath cells : Thick walled, packed tightly all around the vascular bundle, plastids centripetally arranged, lack grana, contain starch grains, absence of PS II, CO2 released by malic acid is fixed by RuBP. Calvin cycle enzymes are present in stroma.
(ii) Mesophyll cells : Concentrically arranged, form most of the leaf tissues; plastids well developed, grana large and well developed; PS II present, CO2 accepted by PEP, CO2 fixation occurs in cytoplasm; Calvin cycle enzymes are absent.
In C4 plants, CO2 is first accepted in the mesophyll cells by phosphoenol pyruvic acid (a highly energetic Cintermediate of glycolysis) using the enzyme PEPCO/PEPCase.
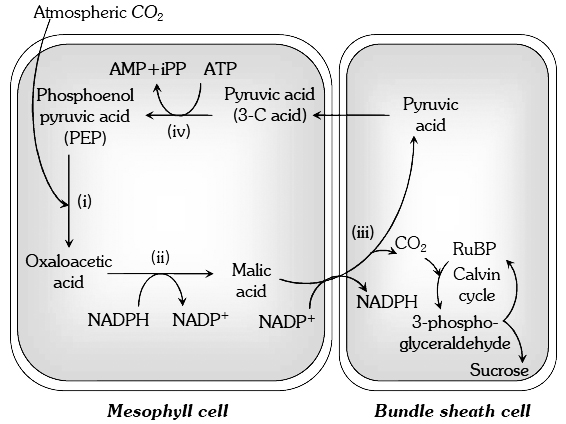
and bundle sheath cells of C4 plants
This forms a C4 compound oxaloacetic acid.
It transforms into another 4 C compound malic acid.
The malic acid now diffuses to the bundle sheath cells where it gives off CO2 It is accepted by ribulose diphosphate and refixed into hexose sugars through Calvin cycle.
The malic acid, after giving off CO2 convert into pyruvic acid and returns to mesophyll cells. It is then converted to phosphoenolpyruvate by using ATP.
This involves formation of AMP (adenosine monophosphate) instead of ADP.
Hence, regeneration of ATP from AMP requires 2 ATP each (12 ATP for formation of 6 molecules of phosphoenolpyruvate, as the 6CO2 molecules are to be fixed).
Therefore, C4 pathway requires 12 additional ATP or total 30 ATP (18 ATP in C3 cycle + 12 additional ATP).
An interesting aspect of C4 cycle is that, it shows a division of labour between two kinds of parenchyma cells showing chloroplast dimorphism -mesophyll chloroplasts act as traps of CO2 which is then (in the form of C4 acids) shuttled to bundle sheath plastids where finally the CO2 is reduced to sugars.
C4 plants are more efficient in picking up CO2 and tolerating drought conditions.
CO2 compensation point C4 is low (0-10 ppm) in C4 plants (as compared to 25-100 ppm) for C3 plants. Photorespiration is absent in C4 plants, because they have a mechanism to concentrate CO2 at enzyme site.
Some major differences between C3 and C4 plants
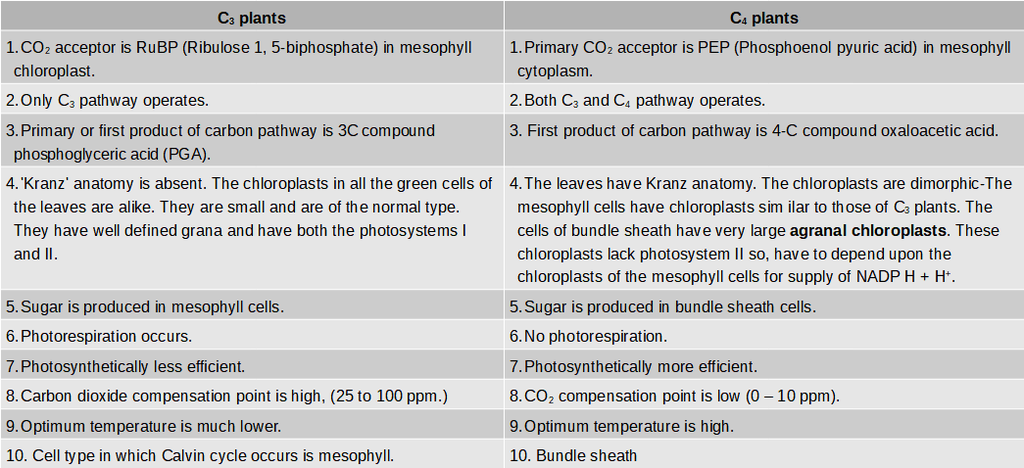
(3) CRASSULACEAN ACID METABOLISM (CAM) (Diurnal acid Cycle
(i) The leaves of plants belonging to Crassulaceae family like Kalanchoe and Sedum undergo crassulacean acid metabolism and such plants are called CAM plants.
(ii) All CAM plants are succulent in habit, including other plants like Orchids, pineapple, certain cactus etc. In these plants, stomata remains open in night and closed during the day (Scotoactive stomata).
(iii) CO2 is fixed during night (dark) to OAA using PEP carboxylase. This CO2 comes from respiration (breakdown of starch) and also from the atmosphere. Malic acid gets stored in vacuoles.
(iv) The CAM plants also contain the enzymes of Calvin cycle. During day time, malic acid breaks into pyruvate and CO2, While CO2 enters the calvin cycle, pyruvate is used up to regenerate PEP.
(v) The succulents, therefore synthesize plenty of organic acids from CO2 during night (when stomata are open) and plenty of carbohydrates during the day (when stomata are closed)(vi) Like Calvin cycle, CAM cycle also operates in the mesophyll ceil. None of these have shown chloroplast dimorphism as is found in C4 plants.
(vii) t should be remembered that the slow growing desert succulents exhibiting CAM cycle have the slowest photosynthetic rate, while the species possessing C4 pathway possess the highest rates.
(viii) Thus CAM plants are although not as efficient as C4 plants, they are definitely better suited to the adverse conditions (i.e., conditions of extreme desiccation).
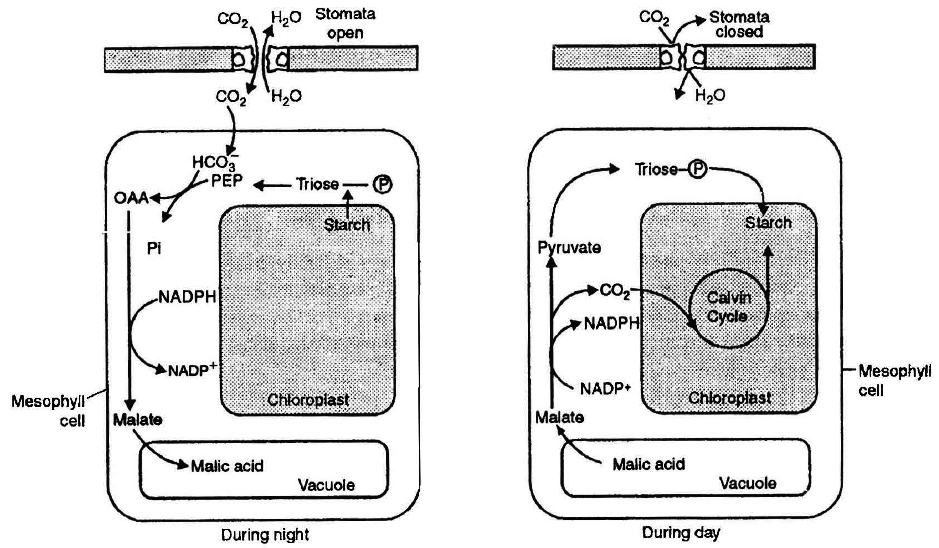
Photorespiration
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PHOTORESPIRATION (C2 Cycle or Glycolate Metabolism)
Term given by (Decker and Tio). Photorespiration is a process which involves oxidation of organic compounds in plants by oxygen in the presence of light.
Like ordinary respiration, this process also releases carbon from organic compounds in the form of carbon dioxide but does not produce ATP.
Thus, apparently it seems to be a wasteful process, but it must have some functions which are still unknown.
The process occurs in C3 plants only.
There are two oxygen consuming reactions in this process.
First oxidation occur in chloroplast and second oxidation occur in peroxisome.
Photorespiration occurs usually when there is high concentration of oxygen.
Under such circumstances RuBP carboxylase (or Rubisco), the enzyme that joins carbon dioxide to RuBP, functions as oxygenase.
As a result, oxygen, instead of carbon dioxide, gets attached to the binding site of the enzyme (i.e. RuBP).
This reaction is competitive.
It is the relative concentration of O2 and CO2 that determines which of the two will bind to the enzyme.
On oxidation, RuBP releases one molecule of C3 compound.
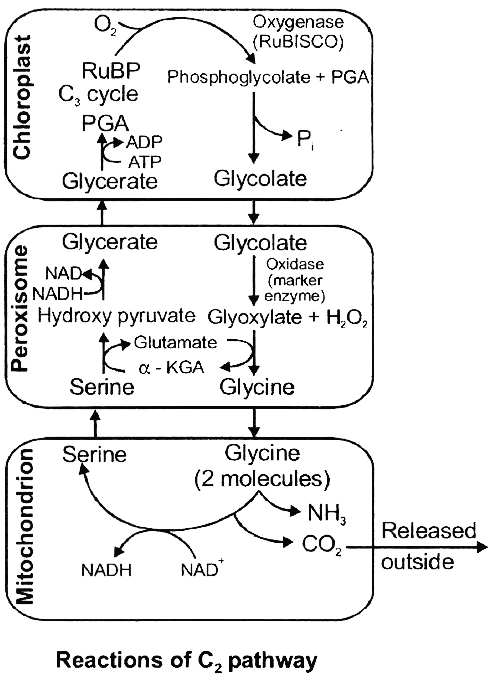
PGA (which enters C3 cycle) and one molecule of a C2 compound phosphoglycolate.
The latter almost immediately changes into glycolate.
The glycolate leaves the chloroplast and enters a membrane bound sac of enzymes called peroxisome.
Here the glycolate is oxidised into glyoxylate which is aminated into glycine.
Further condensation of glycine takes place inside the mitochondria where two molecules of glycine interact and give rise to a molecule of serine and carbon dioxide each.
The CO2 is then released from mitochondria.
Serine regenerates PGA. With increase in temperature, high light intensity and oxygen concentration, affinity of Rubisco for CO2 decreases and its affinity for oxygen increases.
Thus, a rise in temperature means more loss of photosynthetically fixed carbon by photorespiration.
It reduces potential yield of plants growing in the tropics by 30-40%. Upto 1/4th of the photosynthetically fixed CO2 may be lost by this pathway.
Factors affecting Photosynthesis
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Factors affecting Photosynthesis
Photosynthesis occurs under the influence of many factors, both internal and external.
When several factors affect any (bio)chemical process, Blackman's (1905) law of limiting factors comes into effect.
Blackman's Law of Limiting Factors
According to this law "When a process is conditioned as to its rapidity by a number of separate factors, the rate of the process is limited by the pace of the slowest factor."
In other words. "If more than one factors affect a chemical process then its rate will be determined by the factor which is nearest to its minimal value".
It is the factor which directly affects the rate if its quantity is changed.
A limiting factor is that factor which is deficient to such an extent that increase in its value directly increases the rate of the process.
A. External factors affecting photosynthesis
(1) Light :
It is an essential factor for photosynthesis. It affects the rate of photosynthesis in three ways.
I. Light Intensity: It affects the rate of photosynthesis. Optimum value of light intensity is different for different plants.
There are two categories of plants :
(a) Sciophytes : They grow under diffuse light, e.g., Oxalis etc.
(b) Heliophytes: They grow in direct sunlight, e.g., Dalbergia etc.
(i) The optimum light intensity for sciophytes is 10% of full summer sunlight.
(ii) The-optimum light intensity for heliophytes is 50-70% of full summer sunlight.
(iii) Optimum for C4 plants is more than full summer sunlight. e.g., Maize, sugarcane.Optimum light in ensity is also called saturation point.
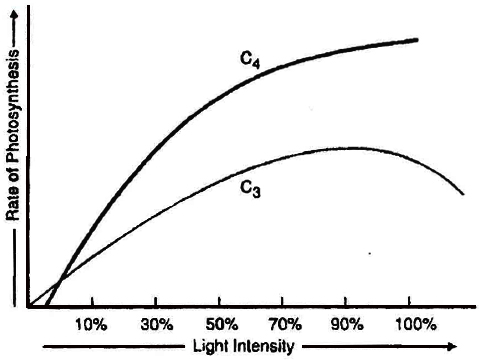
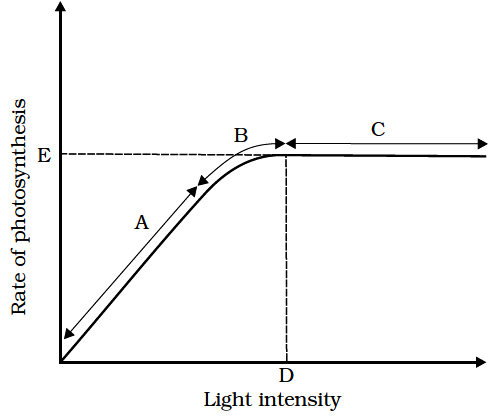
(iv) As the light intensity decreases rate of photosynthesis also decreases. The point in light intensity where there is no apparent exchange of gases between the environment and the photosynthetic organ is called light compensation point. Increase in light intensity from compensation point to saturation point increases the rate of photosynthesis. Light intensity above the saturation point decreases the rate of photosynthesis, this effect is called solarisation. This includes photoinhibition and photo-oxidation.
II. Light quality : Light between 400-700nm wavelength constitute the photosynthetically active radiation or PAR. Maximum photosynthesis takes place when whole white light is available to the plant. Maximum photosynthesis takes place in red and blue light and minimum photosynthesis takes place in green light.
III. Duration of light: Light duration does not affect the rate of photosynthesis, but it affects the total photosynthesis.
(2) Carbon dioxide:
Carbon dioxide concentration is the major factor influencing the rate of photosynthesis.
The CO2 concentration is very low in the atmosphere (between 0.03 and 0.04 percent).
This level of CO2 is far below the requirement for optimum photosynthesis.
Thus, the rate of photosynthesis could be increased several times by increasing CO2 concentration to about 0.05%.
At current level of CO2 concentration the rate of photosynthesis is optimum for C4 plants and lower for C3 plants.
If CO2 concentration increases then only C3 plant would be benefitted. A stage in CO2 concentration when there is no net absorption of CO2 by illuminated plant organ is called CO2 compensation point. At this point, photosynthesis rate is equal to respiration rate. CO2 compensation point for C3 plant is 25-100 ppm and for C4 plant is 0 -10 ppm.
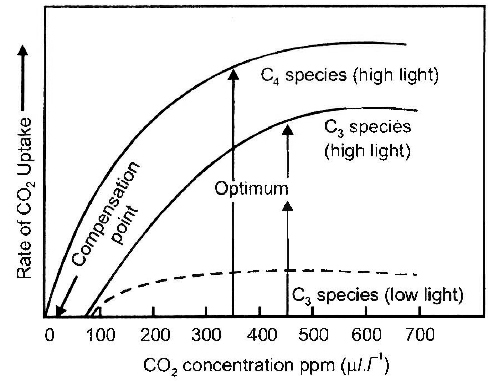
C4 plants show saturation at about 360 ppm, while C3 plants shows this beyond 450 ppm. Thus current CO2 concentration is limiting for C3 plants.
Very high CO2 concentration causes the stomata to close. This inhibits exchange of gases and as a result the photosynthetic rate decreases.
(3) Temperature:
The dark reactions are controlled enzymatically in stroma, hence are temperature regulated.
Optimum value of temperature for photosynthesis in C3 plant is 20-25°C and for C3 plants is 30º–45ºC) The minimum temperature at which most plant starts photosynthesis is 0-5°C. It is however as low as –35°C for some gymnosperms.
(i) Maximum temperature at which photosynthesis can occur is 50-55°C for desert plants (Opuntia) and 70-75°C for hot spring algae.
(ii) Tropical plants have a higher temperature optimum than the plants of temperate climate.
(iii) In C4 plants, pyruvate phosphate dikinase enzyme is sensitive to low temperature, while in C3 plants affinity of RuBisCO for CO2 decreases at high temperature.
(iv) Q10 values for photosynthesis is 2.
(4) Water:
Photosynthetic process utilizes less than 1% of the water absorbed by a plant, hence, it is rarely a limiting factor in photosynthesis.
But water scarcity affects photosynthesis indirectly.
It has been observed in several experiments that the rate of photosynthesis decreases drastically if water supply is withheld for sometime.
Water stress can cause stomatal closing, wilting of leaves and reduced metabolic activities in leave.(5) Oxygen.
Increase in O2 concentration decreases the rate of photosynthesis in C3 plants.
This phenomenon of the reduction of photosynthesis by O2 was first discovered by Warburg using an alga called Chlorella and is called as Warburg's effect.Oxygen is inhibitory because:
(i) It favours photorespiration.
(ii) It destroys the activated state of chlorophyll.
(6) Mineral nutrient.
Few mineral elements are directly involved in photosynthesis. Mg is a component of chlorophyll. Mn, Ca and Cl are essential for photolysis of water.
B. Internal factors affecting photosynthesis
Photosynthesis is under the influence of several internal (plant) factors.
The plant factors include the number, size, age and orientation of leaves, mesophyll cells and chloroplasts, internal CO2 concentration and the amount of chlorophyll.
The plant or internal factors are dependent on the genetic predisposition and the growth of the plant.
(1) Chlorophyll.
Of the internal factors, chlorophyll is the most important because light energy is trapped by only this substance.
There is no photosynthesis in the absence of chlorophyll.
The non green parts of variegated leaves (e.g., Croton), therefore, do not have starch.
Photosynthetic number or assimilation number shows a relationship between the chlorophyll and photosynthesis.
It is the amount of carbon dioxide (in gms) assimilated by one gram of chlorophyll in an hour.
Emerson (1929) observed a direct relationship between the chlorophyll content of a leaf and the rate of photosynthesis.
If all other factors are favourable, increased chlorophyll leads to an increase in photosynthesis.
(2) Photosynthetic products.
With the accumulation of the end products of photosynthesis in mesophyll cells, there is decrease in their photosynthetic rate because concentration of these products in the cells increases the rate of respiration.
Concept Builder
1.'Photosynthesis is an anabolic, endergonic; oxidation-reduction process.
2.Destruction of chlorophyll due to, high light intensity is called solarisation.
3.In C3 plants, more CO2 is released in light than in dark due to photoresprration.
4.Maximum absorption peak of, chlorophyll a and chlorophyll b is in blue light.
5.Chloride ions stimulate quick release of electrons from water.
6.Pheophytin is chlorophyll a ,in which Mg++ is replaced by two hydrogens.
7.Atriplex hastata shows Calvin cycle whereas Atriplex rose us shows Hatch and Slack cycle.
8.The reaction centre in bacteria is B-890 and reducing agent is NADH2, in bacterial photosynthesis, no photolysis of H2O occur, therefore phothesynthesis is an oxygenic and cyclic ETS dominates.
9.Moll's half leaf experiment is used to show that carbon dioxide is essential for photosynthesis.
10.Wilmott's bubbler is used to determine the rate of photosynthesis by counting the O2 bubbles.
Do Plants Breathe
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Do Plants Breathe
Plants require oxygen for respiration to occur and they also give out carbon dioxide . Hence , plants have systems in place that ensure the availability of oxygen . Plants , unlike animals , have no specialised organs for gaseous exchange but they have stomata and lenticels for this purpose .
> There are several reasons why plants can get along without respiratory organs . First , each plant part takes care of its own gas - exchange needs . There is very little transport of gases from one plant part to another . Second , plants do not present great demands for gas exchange . Roots , stems and leaves respire at rates far lower than animals do . Only during photosynthesis large volumes of gases are exchanged and each leaf is well adapted to take care of its own needs during these periods . When cells photosynthesise , availability of oxygen is not a problem in these cells since oxygen is released within the cell . Third , the distance that gases must diffuse even in large , bulky plants is not great . Each living cell in a plant is located quite close to the surface of the plant .
> This is facilitated by the loose packing of parenchyma cells in leaves , stems and roots , which provide an interconnected network spaces The complete combustion of glucose , which produces CO₂ and H₂O as end products , yields energy most of which is given out as heat .
> The strategy that the plant cell uses is to catabolise the glucose molecule in such a way that not all the liberate energy goes out as heat . The key is to oxidise glucose not in one step but in several small steps enabling some steps be just large enough such that the energy released can be coupled to ATP synthesis . During the process of respiratio oxygen is utilised and carbon dioxide , water and energy are released as products .
Glycolysis
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Glycolysis
Most ancient metabolic pathway with the sequence of reactions which converts glucose to pyruvic acid with the production of ATP is termed as glycolysis.
It is an anaerobic process, i.e., it does not require oxygen and is common to both aerobic and anaerobic respiration.
Therefore, it is regarded as the fundamental step in respiratory breakdown of glucose.
It is the only process in respiration among anaerobes.
Glycolysis involves a series of ten reactions, which were elucidated by three scientists - Embden, Meyerhof and Parnas, thus also known as EMP pathway.
These reactions occur in the cytoplasm of cells and do not required oxygen.
The first half of this pathway activates glucose (glucose activation phase).
The second half extracts the energy (energy extraction phase).
Glycolysis yields only 5% of total ATP production and less than 7 % of total energy content of glucose.
Special features of glycolysis can be summarised as follows:
(1) Each molecule of glucose produces 2 molecules of pyruvic acid after partial oxidation, at the end of the glycolysis.
(2) The net gain of ATP in this process is 2 ATP molecules four ATPs are formed in glycolysis but two are already used up in the reaction).
(3) During the conversion of 1,3-diphosphoglyceraldehyde into 1,3-diphosphoglyceric acid, one molecule of NADH2 is formed. As each molecule of glucose yields two molecules of 1, 3-diphosphoglyceric acid, hence, each molecule of glucose forms 2 molecules of NADH2.
(4) During aerobic respiration (when oxygen is available) eachNADH2 forms 3ATP and H2O through electron transport system of mitochondria. In this process 1/2 O2 molecule is utilized for the synthesis of each water molecule.
All the reactions of EMP are reversible except for those catalysed by Hexokinase, Phosphofructokinase and Pyruvic kinase.
Nearly all enzymes require Mg+2 as cofactor.
Brain, retina and skin derive most of their energy from glycolysis.
It is the only source of energy in RBC.
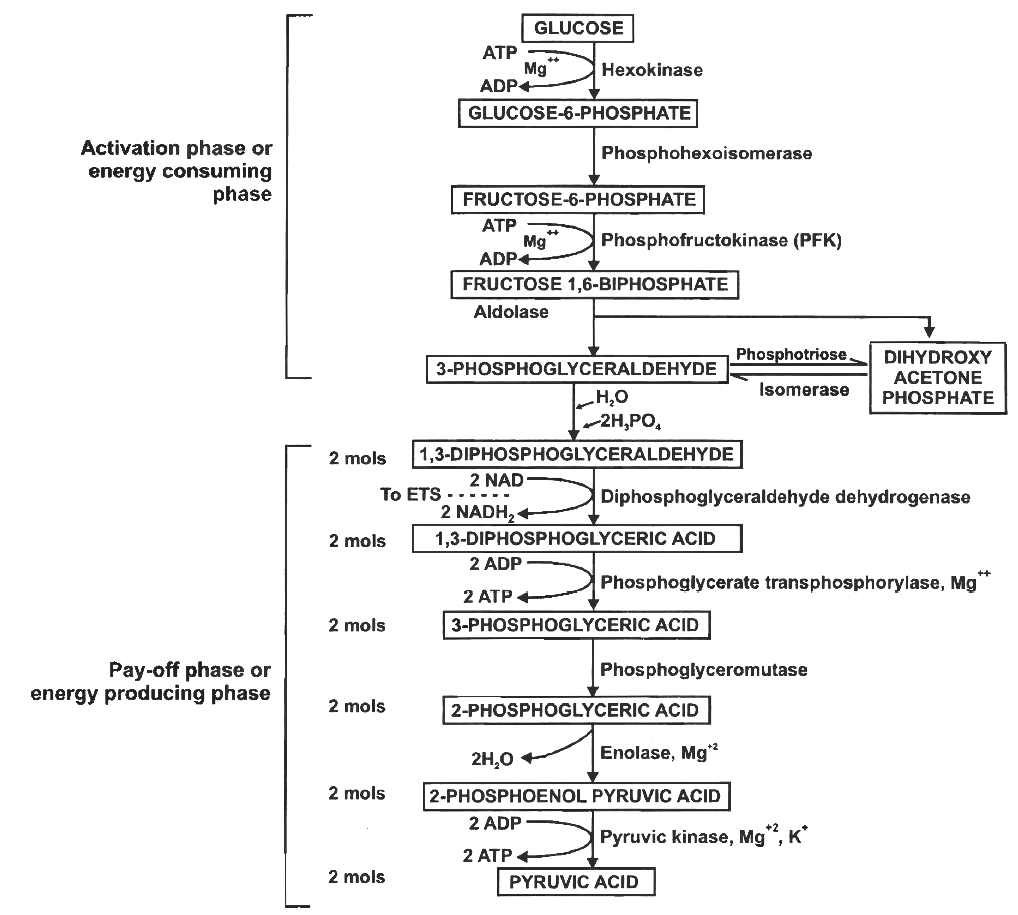
Summary of glycolysis
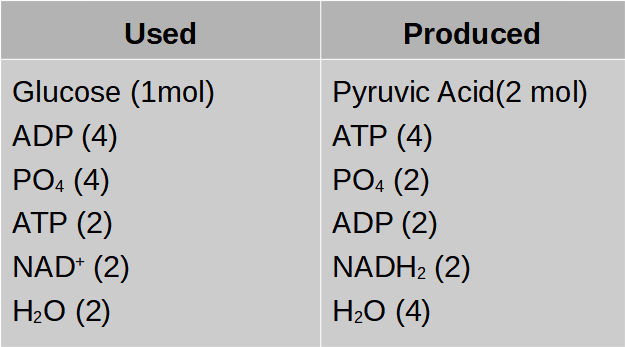
Fermentation
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Fermentation
Fermentation is a kind of anaerobic respiration, carried out primarily by fungi and bacteria.
Although people had been using this process in the preparation of wines since prehistoric times but failed in their attempts to understand the alcoholic fermentation.
Gay Lussac was the first to discover fermentation.
Louis Pasteur concluded that fermentation occurred only when the living yeast cells were present and called this process as zymosis or fermentation.
Fermentation is of following types:
Types of anaerobic respiration and fermentation (Also called intramolecular respiration):
1. Alcoholic fermentation/anaerobic respiration:
It is the most common type of fermentation taking place in yeast and certain bacteria.
Buchner (1897) extracted zymase complex (enzymes) from yeast cells.
Fermentation takes place in the solution of large numbers of sugars, like fructose, galactose, mannose and disaccharides.
In fermentation by yeast, when percentage of alcohol in sugar solution is about 13%, yeast cells are killed and fermentation stops.
At the time of germination of seeds the amount of sugar increases, so that fermentation takes place in germinating grains of barley.
Alcoholic fermentation takes place in two steps.
In the first step, pyruvic acid is decarboxylated resulting in the formation of acetaldehyde and CO2.![]()
In the second step, acetaldehyde is reduced to alcohol by 2NADH + 2H+
![]()
The overall equation is as follows:
![]()
2. Lactic acid fermentation: Pyruvic acid formed at the end of glycolysis is converted to lactic acid by Homofermentative lactic acid bacteria (Lactobacillus lacti)
![]()
3. Lactic acid and ethyl alcohol fermentation
![]()
4. Acetic acid fermentation
(i) ![]()
(ii) ![]()
Comparison of fermentation and aerobic respiration
(i) Partial breakdown of glucose occurs in fermentation, whereas in aerobic respiration it is completely degraded to CO2 and H2O.
(ii) Net gain if only 2ATP molecules in fermentation, whereas it is very high in aerobic pathway.
(iii) NADH is oxidised to NAD+ rather slowly during fermentation, however, the reaction is very vigorous in case of aerobic respiration.
Aerobic Respiration
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Aerobic Respiration
Aerobic respiration is a process in which there is a complete oxidation of organic substances in the presence of oxygen and releases CO₂ , water and a large amount of energy present in the substrate . This type of respiration is most common in higher organisms .
The aerobic respiration takes place in the mitochondria .The final product of glycolysis , pyruvate is transported from the cytoplasm into the mitochondria .
The crucial events in aerobic respiration are :
( i ) The complete oxidation of pyruvate by the stepwise removal of all the hydrogen atoms , leaving three molecules of CO2
( ii ) The passing on of the electrons removed as part of the hydrogen atoms to molecular O₂ with simultaneous synthesis of ATP
The first t process takes place in the matrix of the mitochondria while the second process is located on the inner membrane of the mitochondria .
Pyruvate oxidation : Pyruvate , the end product of glycolysis enters mitochondrial matrix through a specific transport protein . It is decarboxylated oxidatively to produce CO₂ and NADH . The product combines with sulphur containing coenzyme A to form acetyl CoA or activated acetate . The reaction occurs in the presence of an enzyme complex pyruvate dehydrogenase . This step is called gateway step or link reaction because acetyl CoA acts as a connecting link between glycolysis and Krebs ' cycle .
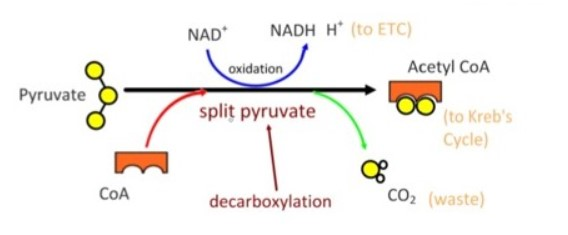
The Respiratory Balance Sheet
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
The Respiratory Balance Sheet
It is possible to make calculations of the net gain of ATP for every glucose molecule oxidised; but in reality this can remain only a theoretical exercise.
These calculations can be made only on certain assumptions that:
There is a sequential, orderly pathway functioning, with one substrate forming the next and with glycolysis, TCA cycle and ETS pathway following one after another.
The NADH synthesised in glycolysis is transferred into the mitochondria and undergoes oxidative phosphorylation.
None of the intermediates in the pathway are utilised to synthesise any other compound.
Only glucose is being respired -no other alternative substrates are entering in the pathway at any of the intermediary stages.
ATP molecules produced during respiration
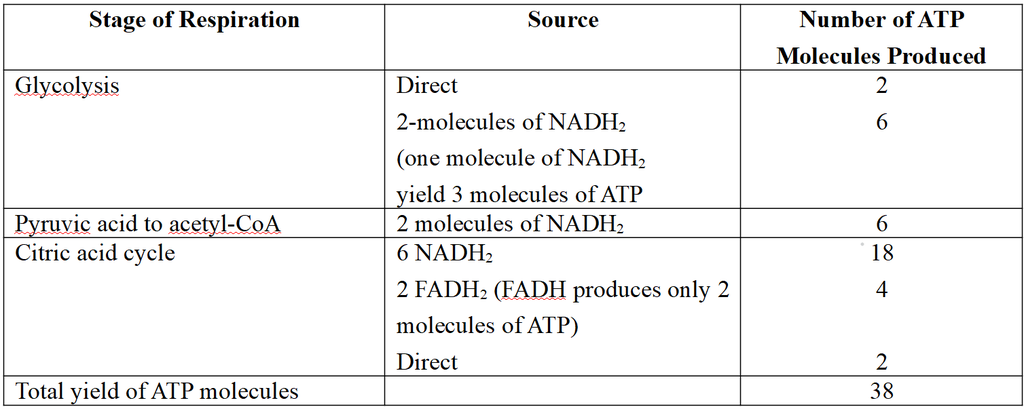
Net Gain of ATP
There is a net gain of 38 ATP molecules during aerobic respiration of one molecule of glucose.
The details of ATP produced have been given in the above table.
In most eukaryotic cells the net gain of ATP is 36 molecules.
Efficiency of Aerobic Respiration
(i) One ATP yields about 34 kJ energy.
(ii) Total energy obtained from 38 ATP moelcule is 1292 kJ. The glucose molecules stores energy of about 2870 kJ.
(iii) Efficiency = ![]() = 45%
= 45%
why respiration is amphibolic?
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Amphibolic Pathway
glucose is the favoured substrate for respiration . All carbohydrates are usually first converted into glucose before they e used for respiration .
Other respired , but they do not enter the respiratory pathway at the first step .
Fats would need to be broken down into glycerol and fatty acids first . If fatty acids were to be respired they would first be degraded to acetyl CoA and enter the pathway . Glycerol would enter the pathway after being converted to PGAL . The proteins would be degraded proteases and the individual amino acids ( after deamination ) depending on their structure would enter the pathway at some stage within the Krebs ' cycle or even as pyruvate or acetyl CoA .
Since respiration involves breakdown of substrates , the respiratory process has traditionally been considered a catabolic process and the respiratory pathway as a catabolic pathway . But this is not completely true .
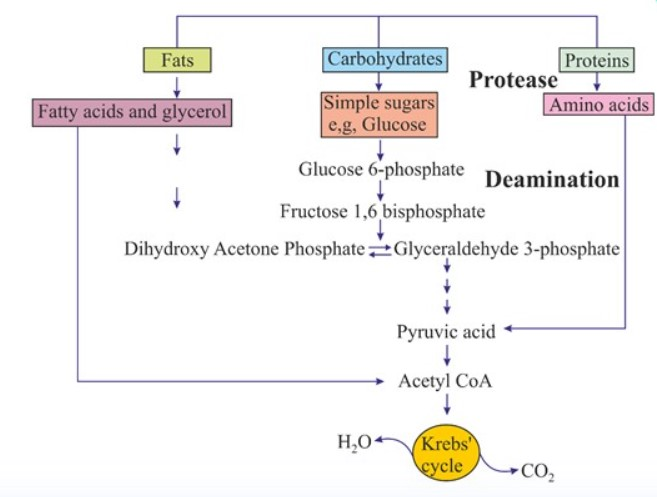
> When the organism needs to synthesise fatty acids , acetyl CoA would be withdrawn from the respiratory pathway for it . Hence , the respiratory pathway comes into the picture both during of fatty acids . Similarly during breakdown and synthesis of protein too , respiratory form the link .
> Breaking down processes within the living organism is catabolism and synthesis is anabolism . Because respiratory pathway is involved in both anabolism and catabolism , it would hence be better to consider the respiratory pathway as an amphibolic pathway .
Respiratory Quotient
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Respiratory Quotient
Respiratory quotient (RQ) or respiratory ratio is the ratio of the volume of carbon dioxide released by a tissue over a period of time to the volume of oxygen absorbed during this period.
RQ = ![]()
The respiratory quotient, besides other factors, depends upon the type of respiratory substrate and the completeness of oxidation.
It can be measured with the help of an apparatus called Ganong's respirometer.
The value of RQ may be equal to unity, more than unity or less than unity, depending upon the substrates oxidised.
This can be illustrated by following examples:
(i) In case of carbohydrates : Plant material where carbohydrates serve as the respiratory substrates (e.g., germinating seeds of cereals, leaves kept in dark, flowers and fruits rich in carbohydrates) have RQ equal to unity, i.e., one.
C6H12O6 + 6O2 ® 6CO2 + 6H2O
RQ = ![]() = 1
= 1
(ii) In case of fats: e.g. , In germinating fatty seeds (e.g., castor, sesame, mustard, linseed, etc.) Fats are poor in structural O2, so the RQ value is less than unity.
2C51 H98O6 + 145O2 ® 102CO2 + 98H2O
(tripalmitin)
RQ = ![]() = 0.7
= 0.7
(iii) In case of proteins -e.g., germinating pulse seeds.
In proteins too, the proportion of carbon is more than oxygen, hence their RQ is also less than unity i.e., (0.5 -0.9).
(iv) In succulents (during night) e.g., Bryophyllum, Opuntia
Incomplete oxidation of carbohydrates occurs during night when stomata are open.
3C6H120 6 + 3O2 —® 3C4H6O5 + 3H2O
RQ = ![]() = 0
= 0
(v) In case of organic acids. In fleshy plants (i.e., members of Cactaceae and Crassulaceae) the organic acids (such as malic acid, oxalic acid, etc.) are produced during night are oxidised during day time. Organic acids contain high proportion of oxygen as compared to carbohydrates, therefore, less oxygen is absorbed than CO2 liberated. The RQ value is more than unity.
e.g., The RQ value is 4 for oxalic acid and 1.33 for malic acid
(vi) In case of'anaerobic condition (in the absence of O2). In the absence of oxygen, tissue respires anaerobically. Thus, CO2 liberation takes place without any utilization of oxygen.
e.g., ![]()
RQ = ![]() = ∞ (Infinite)
= ∞ (Infinite)
(vii) R Q. of maturing fatty seed is > 1.
(viii) RQ. of mixed diet is 0.85.
(ix) RQ. of starved leaves is < 1.
(x) RQ. of coloured petals < 1.
Growth
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PLANT GROWTH
Growth is the essential characteristic of all living organisms.
It can be defined as an irreversible (permanent) increase in mass, weight or volume of cell, organ or organism.
Irreversible increase in size, volume or weight is called apparent growth, as it is external manifestation of growth.
Formation of cellular materials or protoplasm is the real growth.
Growth is a measurable or quantitative phenomenon which can be measured in relation to time.
Growth of living being is internal or intrinsic.
Plant growth is diffused like that of animals only during the early embryonic stages.
Later on, plants develop specific areas, called meristems, for growth.
On account of meristems, plant growth is localised.
Characteristic of Plant Growth
1. Growth is localised.
2. Growth continues throughout life, i.e., indeterminate.
3. There is increase in number of parts.
4. It is open ended.
5. Tile young one or seedling can be quite different from adult.
6. The juvenile stage may have different traits.
Phases of Growth
The dividing or meristematic cells pass through following three phases during growth,
(i) Phase of cell division,
(ii) Cell elongation phase,
(iii) Maturation phase.
(i) Phase of cell division. The meristematic cells located at shoot and root apex are thin walled, densely cytoplasmic and show continuous mitotic divisions. It represents initial stage of growth. The rate of growth is naturally slow during this phase.
(ii) Cell elongation phase. The daughter cells, formed by the division of meristematic cells, undergo enlargement. These can be observed proximal to meristematic cells. Initially, the cells enlarge in all dimensions but subsequently enlargement takes place only in a specific direction. Increased vacuolation and new depositions of wall material are other characteristics to this phase. It is the period of maximum and rapid growth. Physiological activities of cells are at their maximum.
(iii) Maturation or stationary phase. As the cells enlarge, they gradually acquire permanent shapes and forms. The final phase of growth is usually called maturation. As maturation proceeds, the cells become fully differentiated. A mature cell may be living or dead, depending upon the requirements of plant.
Growth curve :
The growth of a plant or any of its organs rarely take place at a constant rate.
There are certain regular changes in rate, that can be observed when growth of any parameter (i.e., weight, volume, size, etc.) is plotted against time.
The curve obtained is characteristically S-shaped. It is called sigmoid curve.
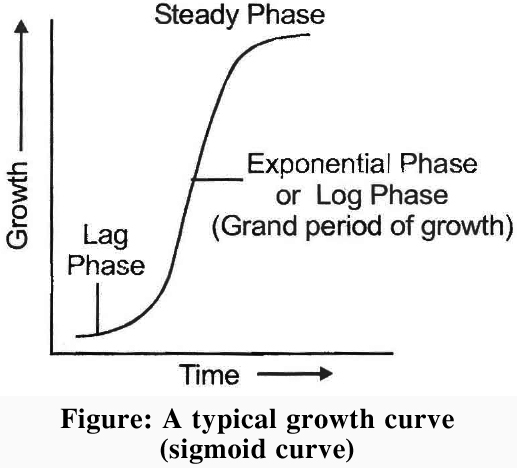
Concept Builder
Measurement of growth:
Growth is measured in terms of increase in length, weight; thickness or volume. Following are some of the methods used to measure growth. Various methods and instruments for growth measurement are listed below:
a. Direct method
b. Horizontal microscope
c. Arc auxanometer
d. Preffer's auxanometer.
e. Crescograph (developed by J.C. Bose) Very sensitive growth measuring instrument which can magnify growth by tenthousandtimes.
Differentiation, Dedifferentiation and redifferentiation
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
DIFFERENTIATION, DEDIFFERENTIATION AND REDIFFERENTIATION
(i) Differentiation: It is permanent qualitative change in structure, chemistry and physiology of cell wall and protoplasm of cells, their tissues and organs.
(ii) Dedifferentiation : It is the process of despecialisation of differentiated living cells so that they regain the capacity to divide and form new cells.
(iii) Redifferentiation: Structural, chemical and physiological specialisation of cells derived from dedifferentiated meristematic cells is called redifferentiation.
Concept Builder
Some examples of differenentiation
(i) Enlargement, lignocellulosic secondary wall thickening and loss of protoplasm in case of tracheary elements.
(ii) Loss of end walls in case of vessel elements.
(iii) Loss of nucleus and perforations in sieve tube members.
(iv) Deposition of suberin and tannins in cells.
(v) Differential wall thickening (in guard cells).
(vi) Secretion of mucilage in root cap.
Development
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
DEVELOPMENT
It is the sequence of changes that occur in the structure and functioning of an organism during its life cycle from seed germination to its death.
Broadly, it is sum total of growth and differentiation.
Plants follow different pathways in response to environment or phases of life to form different kind of structures.
This ability is called plasticity, e.g., heterophylly in cotton, coriander and larkspur.
In such plants, the leaves of the juvenile plant are different in shape from those in mature plants.
On the other hand, difference in shapes of leaves produced in air and those produced in water in buttercup also represent the heterophyllous development due to environment.
Plant Growth Regulators
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PLANT GROWTH REGULATORS
The growth and differentiation of plants is controlled by a special class of chemical compounds called hormones.
These chemicals are synthesized at one place in the plant body and translocated to another where they act in a specific manner.
They are needed in small quantities at very low concentrations.
Plant hormones, also known as growth factors, growth substances, plants growth regulators (PGRs) or phytohormones, can either promote the growth (growth promoters) or may inhibit it (growth inhibitors).
On the basis of their functions in a living plant body, phytohormones can be classified into following groups:
A. Auxins
B. Gibberellins
C. Cytokin ins
D. Ethylene
E. Abscisic acid (ABA)
A. Auxins
Auxins are the best known plant growth regulators.
They have many effects on plant growth.
The earliest experiments on plant hormones were performed by Charles Darwin and his son Francis Darwin.
They worked with the seedlings of canary grass (Phalaris canariensis).
Darwin was amazed to observe that the direction of growth of the coleoptile was influenced only when its tip was exposed to light and not the base where actual growth was taking place.
He further observed that when the coleoptile was illuminated from one side (i.e., unilateral light), it bent towards light, but if the tips were chopped or covered with metal foil, bending response would not occur.
This led Darwin to conclude that certain kind of 'influence' is generated at the tip which is then transmitted to the base (where growth takes place).
However, the influence remained unidentified.
Boysen-Jensen observed that coleoptiles with decapitated tips neither grow, nor bend towards light, (i.e., do not show phototropism).
The normal bending also occurred, if the tip is cut off and replaced with an intervening block of gelatin.
It was thus evident that the influence was due to chemical, diffusing through the block of gelatin.
A. Paal demostrated that coleoptiles would bend even in the dark after certain treatments.
F.W. Went cut off tips of oat coleoptiles and placed them on small agar blocks and let them for a few hours to allow all the 'chemical influence' to diffuse; into the agar.
By this, he isolated auxins in agar block.
He then placed these agar blocks on one side of freshly decapitated coleoptiles.
He observed that the coleoptiles grew and bent away from the side on which the block was placed.
The degree of curvature of the coleoptile was directly proportional to the concentration of the chemical influence in the agar block.
Went named this 'chemical influence' responsible for the phototropic response as auxin (derived from a greek word 'auxein' = to increase or to grow).Hence, Went is credited with the discovery of auxins.
Concept Builder
On the basis of the work done on phototropism so far, following conclusions can be drawn:
(i) Growth regulating auxins are systhesized at the tips (apices) of the plant.
(ii) Auxin synthesis occurs on the illuminated side.
(iii) Auxins are transported from apex to the base or the dark or unilluminated side.
Distribution:
Auxin are synthesized continuously in the shoot and root apices. These are produced in relatively very small amounts in roots. Tryptophan (amino acid) is its precursor.
Auxins from the shoot are transported primarily toward the roots through parenchyma.
This basipetal (from apex to base) movement is called polar transport.
Usually, a low concentration of auxin stimulates the growth, while high concentration would inhibit the growth of the same cell or organ.
Roots, in general, show growth initiation at a very low concentration (0.0001 ppm), while in stem, similar response occurs at a relatively high concentration (10 ppm).
Auxin is active in free state and can be easily extracted. Bound auxin is inactive and meant for storage. e.g., IAA-Aspartic acid, IAA-Inositol, IBA-Alanine.
Chemical nature
Auxins are weak organic acids.
Some auxins occur in plants naturally and are called natural auxins, whereas a large number of synthetic chemicals with similar action are called synthetic auxins.
(a) Natural auxins: The first naturally occuring auxin was isolated by Kogl and Haagensmit (1931) from human urine. It was identified as auxin-a (auxentriolic acid). Later, in 1934 Kogl et. al. obtained another auxin called auxin-b (auxenolinic acid) from corn germ oil (extracted from germinating corn seeds), and heteroauxin from human urine. Heteroauxin, also known as indole-3-acetic acid (IAA), is the best known natural auxin. Besides IAA, Indole Butyric Acid (IBA) is another natural auxin
(b) Synthetic auxins : A number of synthetic compounds showing activities similar to auxins have been produced. Some of these are a-and b -Naphthalene acetic acid (NAA), Phenoxy acetic acid (PAA), 2, 4 -Dichlorophenoxy acetic acid (2, 4-D), 2, 4, 5-Trichlophenoxy acetic acid (2, 4, 5-T), Naphthalene Acetamide (NAAM), ete.
Functions of auxins
(1) Cell enlargement: Auxins cause cell enlargement by solubilisation of carbohydrates, loosening of cell wall microfibrils, synthesis of new wall materia1 and increase in respiration.
(2) Prevention of lodging: Lower internodes of the stem of cereals are long and weak. As a result the plant bends down or droops (a process known as lodging). Application of NAAM prevents lodging.
(3) Apical dominance: In many plants, apical bud suppresses the growth of lateral buds. This condition is known as apical dominance. A plant with strong apical dominance has little or no branching (e.g., sunflower).
Pruning (removal of shoot tips) of trees, tea plantations and hedge making is actually done to remove apical buds so that lateral buds may be relieved of their suppression. It results in dense growth of hedges.
(4) Responsible for phototropism and geotropism.
(5) Promote root initiation in callus and stem cuttings.
(6) Delay of abscission of leaves by preventing formation of abscission layer. But promotes this in older leaves and fruits.
(7) Induce parthenocarpy (production of seed less fruits e.g., Tomato).
(8) Have feminising effect (increase number of female flowers in plants e.g., Cannabis).
(9) Seasonal activity of cambium is promoted by auxin.
(10) Healing of injury is affected through auxin-induced division in cells around injured area.
(11) Auxin induces negative potential in cell membranes.
(12) Promotes xylem differentiation.
Applications of Synthetic Auxins
1. Rooting in stem cuttings: IBA, IBA-alanine and NAA are used.
2. Parthenocarpy : IAA, IBA.
3. Weedicide : 2,4-D, 2,4,5-T are used for killing broad leaved weeds (generally dicot).
4. Flowering: NAA and 2,4-D for litchi and pineapple.
5. Storage : Methyl ester of NAA for storage of potato.
6. Preharvest fruit drop: 2,4-D for Citrus fruits, NAA for tomato.
7. Vegetable crops: Chlorophenoxypropionic acid is used to improve quality of vegetable crops by inhibiting flower formation. e.g., lettuce.
8. For Dwarf shoots: NAA is used for increasing dwarf shoots and number of fruits in apple.
Concept Builder
Antiauxins : They inhibit aaxin activity.
e.g., TIBA (Triiodobenzoic acid)
PCIB (p-chlorophenoxy isobutyric acid)
Bioassay of Auxin
(i) Avena curvature test
(ii) Split pea test
(iii) Root growth inhibition test
B. Gibberellins
Gibberellins are another important group of growth promoting hormones.
They stimulate growth largely in the shoot system of the plants and have little or no effect on roots.
Japanese rice farmers observed some abnormally tall and thin seedlings in their rice fields which never bore seeds.
This disease was called "bakanae" or "foolish seedling disease".
E. Kurosawa found that the causative agent of the disease was Gibberella fujikuroi, a fungus of the class Ascomycetes.
He produced the same effect on rice plants with an extract of this fungus.
Later, Yabuta and Sumiki isolated the substance responsible for rapid growth from the extract of the fungus and it was named gibberellic acid (GA). Commercial production of GA is still carried out by culturing this fungus in large vats. Fusarium moniliforme is the imperfect stage of this fungus.
Distribution : Gibberellins are found in almost all groups of plants such as algae, fungi, mosses, ferns, gymnosperms and angiosperms. Gibberellins and gibberellin like substances have been observed in most of the higher plants.
Types of gibberellins : Gibberellins are synthesised in the apices of young leaves, embryo, buds and roots and are transported through xylem. There are more than 100 types of gibberellins reported from widely differnt organisms. They are denoted as GA, GA2 and so on. However, GA3 was one of the first gibberellin to be discovered and remains the most intensively studied, common form.
Chemically, II gibberellins are terpenes, a complex group of plant chemicals related to lipids. All are weak acids and have gibbane ring skeleton. Precursor is Acetyl CoA.
Functions of Gibberellins
(1) In rosette forming plants, (e.g. , cabbage, henbane, radish, beet) internode growth is poor but large leaves appear to arise in tufts. The internodes suddenly elongate and the stem becomes normal just before flowering. This is called bolting. This requires long day conditions and gibberellin treatment.
(2) Substitution of cold treatment : Biennial plants flower only when they receive low temperature during winter season. Such plants would, however, flower after gibberellin treatment even if they do not receive suitable low temperature. Thus, biennial plants can be made to flower in a single year by gibberellin treatment.
(3) Parthenocarpy : Gibberellins have been found to be effective in inducing parthenocarpy in tomato, apple, pear,-etc.
(4) Breaking of dormancy: Gibberellins can effectively break the dormancy of potato tubers, winter buds and seeds of many trees. Gibberellins thus also act antagonistically to abscisic acid (ABA).
(5) Increase in fruit size: Gibberellins increases the number and fruit size of grapes {pomalin (GA + BAP) is used for this purpose}.
(6) Production of male flowers : Gibberellins induce production of male flowers on genetically female plants of Cannabis and cucumber. They promote flowering in LDP, even in non-inductive periods.
(7) Internodal elongation : Like auxins, the main effect of gibberellins is on stem elongation. Gibberellins stimulate stem elongation and leaf expansion, but do not affect roots. Thus, Gibberellins restore normal size and growth in genetically dwarf varieties of pear and maize.
(8) Germination of seeds: Promoted (especially in cereals).
(9) Vernalization: Gibberellins can substitute vernalization demands.
Commercial Application:
1. Increasing length of grape stalks.
2. Malt : Increase yield of malt from barley.
3. Overcoming dormancy: In photoblastic seeds of tobacco and lettuce.
4. Delayed ripening : In Citrus.
5. Increase in length of sugarcane, hence yield.
6. Early seed production can be induced in conifers by treating them with GA in juvenile period.
Concept Builder
Antigibberellins : Certain chemicals are antagonistic to gibberellins.
e.g., Phasphan-D,
Amo - 1618
CCC (Chloro Chaline Chloride)
Maleic hydrazide (MH).
Bioassay of Gibberellins
(i) Induction of -amylase in barley endosperm test.
(ii) Dwarf maize test.
(iii) Dwarf pea test.
C. Cytokinins
Skoog et. al. observed that callus formation from internodal segments tobacco can proliferate, only if auxins are also supplied with (a) extract of vascular tissue (b) yeast extract (c) coconut milk or (d) DNA.
Later, Miller et. al. showed that yeast extract contained some growth regulator.
Finally, this growth regulator was isolated from herring sperm DNA and yeast cells and was named kinetin (6-furfurylaminopurine).
The name cytokinin was adopted by Letham (1963).
The first naturally occurring cytokinin to be chemically identified was from young maize (Zea mays) grains.
Hence it was named zeatin.
Similar form was isolated from coconut milk (liquid endosperm of coconut).
Distribution: Cytokinins are most abundant in the tissue where rapid cell divisions occur, like developing fruits, root apices and developing shoot buds are particularly rich in cytokinins.
Functions of Cytokinins
(i) Cell-division: Cytokinins are quite abundant wherever rapid cell division occurs, especially in growing tissues.
(ii) Morphogenesis: Cytokinins promote cell division. In the presence of auxins, cytokinins promote cell division even in non meristematic tissues. In tissue cultures, mitotic divisions are accelerated when both auxin and cytokinin are present. The ratio of high cytokinin to low auxin promote shoot buds in tissue culture.
(iii) Apical dominance: Cytokinins and auxins act antagonistically in the control of apical dominance.
(iv) Delay in senescence: Cytokinins delay the senescence (Richmond Lang effect) of plant organs by controlling protein synthesis and mobilisation of resources. These help to produce chloroplast in leaves. Hence, these are also called antiageing hormones.
(v) Flowering : Cytokinins also induce flowering in certain species of plants such as Lemna and Wolffia and are also responsible for breaking the dormancy of seeds of some plants.
(vi) Phloem transport is promoted.
(vii) Accumulation of salts in the cells is promoted.
(viii) Sex-expression: Promote femaleness.
(ix) Increases resistance to low and high temperature and diseases.
Commercial application:
(a) Tissue culture.
(b) Shelf life of vegetables and cut flowers is increased .
(c) Overcoming senescence.
Concept Builder
Bioassay:
(i) Chlorophyll preservation test.
(ii) Cell division test
D. Ethylene
Ethylene is the only gaseous PGR and is effective in conc. 0.01 to 10 ppm.
It is produced by most or all plant organs but maximum production occurs in ripening fruits and senescent leaves.
High concentration of auxin induce the formation of ethylene. Its precursor is methionine.
This hormone could fit in both categories, i.e., inhibitor and promotor, but it is largely a growth inhibitor.
Unlike other plant hormones, ethylene is a gas at normal temperature and pressure.
Thus it travels not only within plant in the intercellular spaces, but also through the air, from site of its production to its target tissue which may be part of the same plant or of another plant.
Like, ethylene given out by apples inhibits the sprouting of potatoes stored in the same godown.
Role of ethylene : It regulates so many physiological processes, hence is one of the most widely used PGR in agriculture.
(i) Inhibition of stem elongation and stimulation of transverse growth : Ethylene causes increase in the girth of the plant, and promotes horizontal growth.
(ii) Fruit ripening:
Ethylene is known chiefly for its effects on fruit ripening. Hence, it is used on a commercial scale to stimulate ripening of bananas, apples, mangoes, citrus fruits, tomatoes and many other fruits. These fruits are picked up before ripening and subsequently ripening can be regulated with ethylene when needed. The ripening of fruits by ethylene is accompanied by increase in their rate of respiration. This is known as respiratory climacteric effect.
Ethylene production by plants is autocatalytic, i.e., a small amount of ethylene will stimulate production of its larger amounts. Thus, a few ripe fruits will initiate ripening of nearby fruits. It is, therefore, a common practice to keep few ripe bananas with unripe bananas to hasten ripening.
(iii) It promotes apogeotropism in roots. Seedlings develops epicotyl hook.
(iv) Senescence : It increases senescence of leaves and flowers.
(v) Abscission : It induces abscission of leaves, flowers and fruits.
(vi) Breaking of dormancy : It breaks the dormancy of storage organs, and initiates germination in peanut seeds
(vii) Root initiation: Low concentration of ethylene induces rooting, growth of lateral roots and root hairs.
(viii) Flowering: Ethylene is used to initiate flowering and synchronising fruit set in pineapples.
(ix) Sex expression: Ethylene, like auxins has a feminising effect on sex expression of genetically male plants of Cannabis and monoecious plants of cucumber.
Applications
1. Ethylene in the name of ethephon is used to hasten ripening tomatoes and apples, and to accelerate abscission in flowers and fruits (thinning of cherry, walnut and cotton).
2. Increasing the number of female flowers and fruits in cucumber.
3. Sprouting of storage organs - rhizomes, tubers and other storage organs can be made to sprout by ethylene.
Bioassay of ethylene : Triple response test.
E. Abscisic Acid
In contrast to auxins, gibberellins and cytokinins which stimulate growth, abscisic acid is chiefly a growth inhibitor.
It was discovered almost simultaneously by Addicott (1963) and Wareing (1964).
They named it abscisin II and dormin respectively.
In 1967, it was decided to call it abscisic acid (ABA).
Since then, it has been found in all groups of plants (from mosses to higher plants).
In liverworts and algae, a compound lunularic acid has been found having activities similar to ABA.
Chemically, ABA is a dextro-rotatory cis-sesquiterpene.
ABA is a major inhibitor of growth in plants and is antagonistic to all the three growth promoters, especially gibberellic acid.
Its precursor is violaxanthin (a xanthophyll in chloroplast)
Abscissic acid is produced mainly in mature leaves.
Besides, it is also synthesized in stems, fruits and seeds and then transported to the rest of the plant through vascular tissue. Some of the important roles of abscisic acid in plant growth are as follows.
Bioassay of ABA: Cotton or Bean explant test.
Functions of abscisic acid
(i) Abscission : It hastens the formation of abscission layer and senescence.
(ii) Transpiration: It helps in closing of stomata by causing potassium ions to leave the guard cells (thus reducing their turgidity) during periods of water shortage or drought and, hence is also known as stress hormone.
(iii) Promotes bud dormancy during winters.
(iv) Seed dormancy : ABA induces seeds dormancy, hence is named dormin, thus it helps the seed to withstand desiccation and other unfavourable factors.
(v) Stoppage of cambial activity : Abscisic acid inhibits cambial activity.
(vi) Flowering: ABA induces flowering in some short day plants like strawberry and black currants.
(vii) It plays an important role in seed development, maturation and dormancy.
(viii) Induces synthesis of carotenoids in green oranges making them yellow.
Photoperiodism
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PHOTOPERIODISM (Term by Garner and Allard)
The response of plants to changes in the relative lengths of day and night is called photoperiodism.
Photoperiod
The relative lengths of dark and light periods in a day vary from place to place and from season to season. The length of light period is called photoperiod. At equators, day length is of 12 hours duration throughout the year. Plants flower only when exposed more or less than a certain light period called critical photoperiod.
Types of plants according to photoperiodic requirements for flowering :
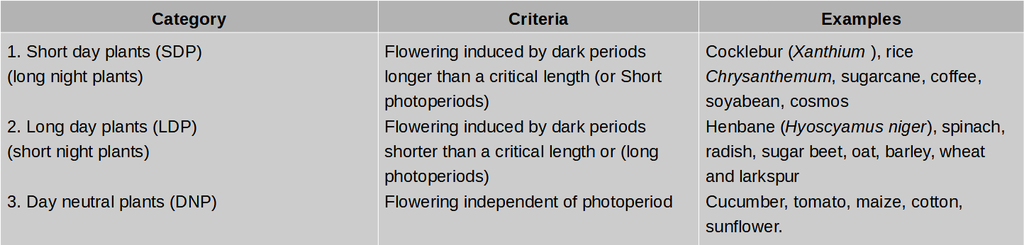
For SDP![]() while for LDP
while for LDP ![]() is critical for flowering
is critical for flowering
Photoperiodic stimulus is perceived by leaf.
When proper photoperiod is perceived a flowering hormone called florigen is synthesized in leaf and is transported to bud through phloem, where flowering occurs.
Florigen is a hypothetical hormone and is chemically similar to gibberellins.

Vernalisation
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
VERNALISATION
There are plants, in which flowering is qualitatively or quantitatively dependent on exposure of low temperature.
In annual plants growth usually starts in the spring, flowers are formed in the summer and fruits and seeds are produced in the fall.
Biennials on the other hand, remain vegetative in the first growing season and after prolonged exposure to cold winter temperature, flower in the following season.
Cold treatment during winters is essential for flowering for these plants.
If they do not get it, they either do not flower or their flowering is much reduced.
The effect of cold or chilling treatment that plants get during winters in the field can be given in the laboratory.
As a result of this artificial cold treatment, the biennials and many winter varieties of cereals can be made to flower in the same season.
This low temperature treatment is known as vernalization or yarovization (= making spring like).
The term vernalization was given by Russian scientist Lysenko.
Site of vernalisation : Shoot tip, embryo tip, root apex, as observed by Wellensiek.
Requirements of Vernalisation
1. Low temperature : 0º -5ºC
2. Period of low temperature : Few hours -few days
3. Actively dividing cells (Meristematic cells)
4. Water
5. Aerobic condition
6. Proper nourishment
In the process of low temperature treatment a hypothetical hormone called vernalin (by Melcher's) is produced.
It is now believed that vernalin is a gibberellin like substance, because vernalized plants have higher gibberellin levels than non vernalized plants.
Vernalization is beneficial in reducing the period between germination and flowering.
Thus, more than one crop can be obained during a year.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
