Cell cycle
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
CELL CYCLE AND ITS PHASES
The total duration of cell cycle varies from organism to organism and also from cell type to cell type.
Yeast for example, can progress through the cell cycle in only about 90 minutes.
The cell cycle is divided into two basic phases :
1. Interphase
2. M-Phase (Mitosis phase)
1. Interphase :
It is also called as preparatory phase and a period of great metabolic activity.
It is the stage, between two successive cell divisions in which no division of chromosomes or cytoplasm occurs.
'In this stage the nucleus and cytoplasm remain metabolically and synthetically very active.
It generally covers over 95% of the total duration of cell cycle.
During this phase, replication of DNA, synthesis of nuclear histones, division of centrioles to form a new pair of centrioles, synthesis of energy rich compounds, RNA and proteins occur. Nuclear envelope remains intact.
Chromosomes occur in the form of long, coiled, indistinctly visible chromatin fibres.
The size of nucleolus is greatly increased due to accumulation of rRNA and ribosomal proteins.
Interphase is divided into three phases:
(a) G1 Phase (b) S or Synthetic Phase
(c) G2 Phase
(a) G1 Phase (Post-mitotic gap phase) : It corresponds to the interval between mitosis and initiation of DNA replication. Following biochemical changes occur during this sub-stage.
(i) The cell grows to its maximum size due to normal metabolic activity for the preparation of DNA replication, but no change occurs in the DNA contents of the cell.
(ii) It undergoes synthesis of new proteins and RNA. Transcription of rRNA, tRNA and mRNA occurs during this phase.
(iii) Nucleotides, amino acids and energy rich compounds (e.g., ATP) are formed.
(iv) It takes maximum time of all the stages. It is most variable in length, due to which time of cell division differs in cell to cell. G1 can be terminated by various stimuli, but once a cell has completed G1 and entered the 'S' phase to start replication of DNA, it cannot be terminated.
(v) Some cells in the adult animals do not appear to exhibit division (e.g., heart cells) and many other cells divide only occasionally, as needed to replace cells that have been lost because of injury or cell death. These cells do not divide further, exit G1 phase to enter an inactive stage called quiescent stage (G0) of the cell cycle. Cells in this stage remain metabolically active, but no longer proliferate unless called on to do so, depending upon the requirement of the organism. Hence, this exit may be temporary or permanent.
Antephase is the end of G1 when the cell reaches a stage whereby, it will divide even under stress condition.
(b) S or Synthetic Phase
(i) In this phase, the synthesis or replication of DNA occurs on the template of existing DNA.
(ii) During this phase, the amount of DNA per cell doubles (means the organism will have duplicate set of genes). However, there is no increase in the chromosome number (ploidy level remains same). If the initial amount of DNA is denoted as 2C then, it increases to 4C, and if the cell had 2n number of chromosomes at G1, even after S phase the number remains same, i.e., 2n.
(iii) In animal cells, the replication occurs in nucleus, and the centriole duplicates in cytoplasm.
(iv) Histone proteins are synthesised in S-phase. S-phase is called invisible phase of cell cycle as replicated chromosomes are not visible at this stage.
(c) G2 Phase (pre-mitotic gap phase)
(i) In this phase, the cytoplasmic organelles such as mitochondria, chloroplast and golgi complex are doubled.
(ii) Synthesis of RNA and protein continues. Spindle protein (tubulin) synthesis and aster formation occurs.
(iii) A cell contains double the amount (4C) of DNA present in the original diploid (2N) cell.
(iv) The cell prepares itself to enter into "M" or Mitotic phase.
(v) It is also signified by the synthesis of some protein kinases for regulation of cell division.
2. M-Phase :
It represents the phase when the actual cell division or mitosis occurs.
It starts with the nuclear division, corresponding to the separation of daughter chromosomes (Karyokinesis) and usually ends with division of cytoplasm (Cytokinesis).
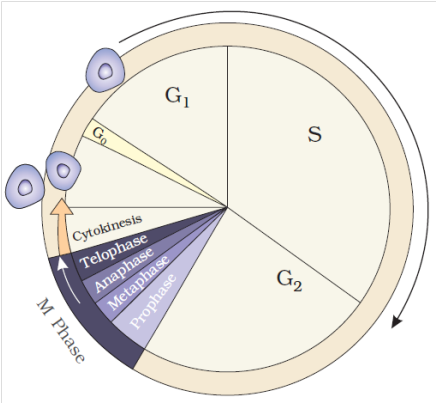
A diagrammatic view of cell cycle indicating formation of two cells from one cell
Regulation of Cell Cycle
Decision of a cell to divide occurs in G1 phase. If a cell is not to divide it will enter into G0 phase or Quiescent phase. When the conditions change, the cell can enter back into G1 phase. G1 S transition in the cell cycle is called as Restriction point or check point. This is the major check point. Once the cell crosses the restriction point rest of the cell cycle is completed. Another minor check point is G2 M transition.
Concept Builder
(i) Cell cycle is regulated by cyclin-dependent protein kinase.
(ii) Cyclins are proteins that activate protein kinases to regulate eukaryotic cell cycle.
(iii) G1 to S transition is carried out by G1 cyclin + cdc 2 kinase.
(iv) G2 to M transition is triggered by maturation promoting factor (MPF) formed by mitotic cyclin + cdc2 kinase, Nucleus attains the maximum size.
(v) The factors which determine whether a cell has to divided or not are
(a) Surface area: Volume ratio. A cell should have high surface area : volume ratio.
(b) Karyoplasmic index.
(vi) Onion root tips or other meristematic tissues are used to study mitosis.
(vii) Mitogens are substances which induce mitosis. e.g., Auxin, Cytokinin, Gibberellin, Insulin etc.
(viii) In animal cell, mitosis is called as Amphiastral (Spindle is associated with 2 asters).
(ix) In plant cells, the mitosis is called as Anastral (no aster, no centriole).
(x) If mitosis is extranuclear, it is Eumitosis.
(xi) If mitosis is intranuclear, it is called as Premitosis. If centrioles are present then it is called as centric.
The cell division is of three types
I. Mitosis II. Meiosis III. Amitosis
Cell cycle
Chapter 10
Cell Cycle and cell division
Cell Cycle
Cells, and all living things for that matter, exhibit growth and reproduction. Each parental cell produces two daughter cells every time it divides, which is how all cells reproduce. A new cell population can be created by the growth and division of a single parental cell and its offspring, which is accomplished by the newly generated daughter cells. In other words, repeated cycles of growth and division enable the formation of structures made up of millions of cells from a single cell.
All living things go through the process of cell division. DNA replication and cell proliferation also happen when a cell divides. To ensure proper division and the production of offspring cells with complete genomes, processes like cell division, DNA replication, and cell development are coordinated. Cell cycle refers to the series of actions that a cell takes to reproduce its genome, synthesise the other components of the cell, and ultimately divide into two daughter cells. DNA synthesis only takes place during one particular stage of the cell cycle, despite the fact that cell growth (as measured by cytoplasmic expansion) is a constant process.During cell division, a complicated chain of processes transfers the replicated chromosomes (DNA) to the daughter nuclei. These occurrences are genetically determined.
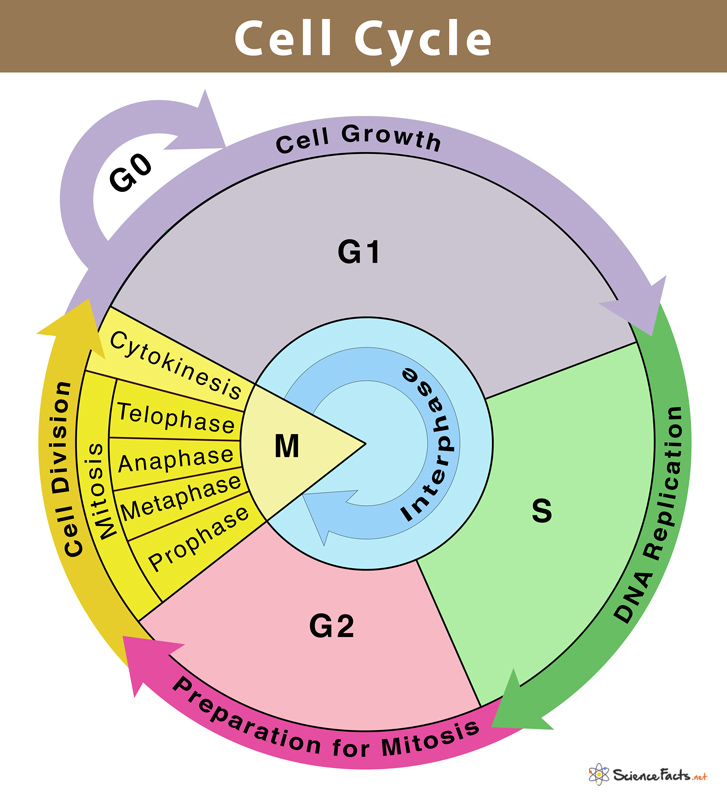
In order to form two genetically identical cells, cells go through a series of carefully timed and regulated steps of growth, DNA replication, and division. Interphase and the mitotic phase are the two main stages of the cell cycle. The cell develops and DNA replication occurs during interphase. The cell divides and the replicated DNA and cytoplasm are separated during the mitotic phase.
The replication and reproduction of cells, whether in eukaryotes or prokaryotes, happens during the cell cycle. Although it serves several purposes for organisms, it ultimately ensures their survival. Prokaryotes can continue to exist by dividing into two new daughter cells thanks to a process termed binary fission in the cell cycle.
Reproduction, growth, and gamete creation are the three primary purposes of cell division. For asexual reproduction, growth, repair, and regeneration, mitosis is necessary. The bodies must create new cells—and permit the death of old cells—in order to expand and develop. The process of healing an injury also requires cell division.If cells were unable to divide and produce new cells, living organisms would never be able to regenerate skin cells to treat rashes or regrow a fingernail.
What is a cell ?
Chapter 8: Cell the unit of life
1. What is a Cell?
All living organisms are composed of cells. Cells are the building blocks of any living body. It is the basic structural as well as the functional unit of life. Some living organisms are made up of only a single cell and are thus known as unicellular organisms (e.g. bacteria), while many other organisms are composed of numerous cells and are therefore called multicellular organisms (e.g. plants and animals).
The invention of the microscope in the 17th century paved the way for the discovery of cells. Thecell was firstdiscovered by Dutch scientist Antonie van Leeuwenhoek. He was the first person to observe and describe a live bacterial cell. He is also known as the father of microbiology. Later, the discovery of the nucleus in a cell, by Robert Brown was another significant milestone in the field of microbiology.
What is a cell ?
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
LIVING CELL
Unicellular organisms are capable of (i) independent existence and (ii) performing the essential functions of life. Hence, cell is the fundamental structural and functional unit of all living organisms.
HISTORICAL ACCOUNT
Aristotle proposed that all animals and plants, however complicated but are constituted of a few elements which are repeated in each of them.
The simple microscope was invented by Galileo.
The first compound microscope was made by Robert Hooke (1665).
He examined thin slices of cork under his microscope and observed the honey comb like structures composed of box like compartments which were termed as the cellulae (cells).
His work was published in his book "Micrographia". Cells were observed prior to Hooke by Malpighi (1661), who called them saccules and utricles.
Leeuwenhoek observed few living cells capable of moving, such as bacteria. protozoa, spermatozoa and red blood corpuscles under his own designed microscope.
Lamarck remarked that "no living being can have life if its constituent parts are not formed by cells".
Dutrochet concluded that plants and animals were made up of globular cells and the cells are held together by cohesion.
In 1831, Robert Brown discovered the presence of nucleus in the cells of orchid root.
Fontana discovered nucleolus in the skin cell of Eel.
The term nucleolus was given by Bowman.
Colloidal theory of protoplasm explains the nature of protoplasm in the best manner. It is most acceptable theory.
It was proposed by Fischer, according to which conversion of solution into gel and vice versa is due to colloidal nature of cytoplasm.
Presently, this can be better explained as "Multiphasic colloidal system of life".
Protoplasm theory was proposed by Max Shultze (1861).
According to it "cell is an accumulation of living substances which is limited by an outer membrane & possesses a nucleus".
M.J. Schleiden, a German botanist in 1838 stated that "All plants are formed of one or more cells".
Theodore Schwann, a German Zoologist in 1839 stated that "All animals are formed of cells, have nuclei and are enclosed by thin cell membrane instead of thick cell wall as found in plant cells".
Schwann proposed the hypothesis that the bodies of animals and plants are composed of cells and their products.
Rudolf Virchow in 1858 observed that new cells arise from pre-existing cells by division i.e., Omnis cellula e cellula.
Cell Theory
Cell Theory
In the year 1838, a German botanist called Matthias Schleiden discovered that all plant tissues are made up of different types of cells. A British Zoologist, Theodore Schwann, who was a contemporary of Schleiden, reported the presence of athin outer layer in the animal cells which is now known as the plasma membrane. Schwann also discovered that along with plasma membrane,an additional unique outer layer is also found exclusively in plant cells i.e. the cell wall. Both Schleiden and Schwann together postulated the Cell Theory, based on their findings. However, this theory did not give any indication of the genesis of cells.
In 1855, a German biologist named Rudolf Virchow first explained the origin of cells from pre-existing cells. This led to the modification of the original cell theory proposed by Schleiden and Schwann which is today understood as:
- All living organisms are made up of cells and cell products.
- Every cell is made up of pre-existing cells.
Cell Theory
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
CELL THEORY
The theory was formulated by Schleiden and Schwann.
The various points of cell theory are:
1. Each cell is made of a small mass of protoplasm having a nucleus and bounded by a cell membrane with or without cell wall.
2. All cells are basically alike in structure and metabolism.
3. Organisms are composed of cells and their products.
4. The functions of an organism is an outcome of activities and interactions of its constituent cells.
But, it did not explain as to how new cells are formed.
Cell theory was first modified in the light of Virchow's findings that cells develop from pre-existing cells i.e.,"Omnis cellula e cellula". It is known as law of cell lineage. Number of other modifications were carried out in cell theory. The modern cell theory is known as cell principle or cell doctrine.
Drawbacks of Cell Theory
Important drawbacks of cell theory are given below:
1. Viruses cannot be explained using this theory.
2. Bacteria and blue-green algae do not have an organized nucleus.
3. Certain fungi, such as Rhizopus, have hyphae composed of a multi-nucleated mass of cytoplasm (coenocyte).
4. Acetabularia (unicelled, marine green algae) has a uninucleated differentiated body (acellular).
5. Sieve tube and mature RBC lack nuclei.
6. Volume of the cell is occupied by a semi-fluid matrix called cytoplasm, and is main arena of cellular activities in both pro and eukaryota.
7. Cells differ greatly in size, shape and activities e.g., Mycoplasma -the smallest cells (0.3 µm length), Bacteria (3 -5 µm), Human RBC (~7.0 µm)
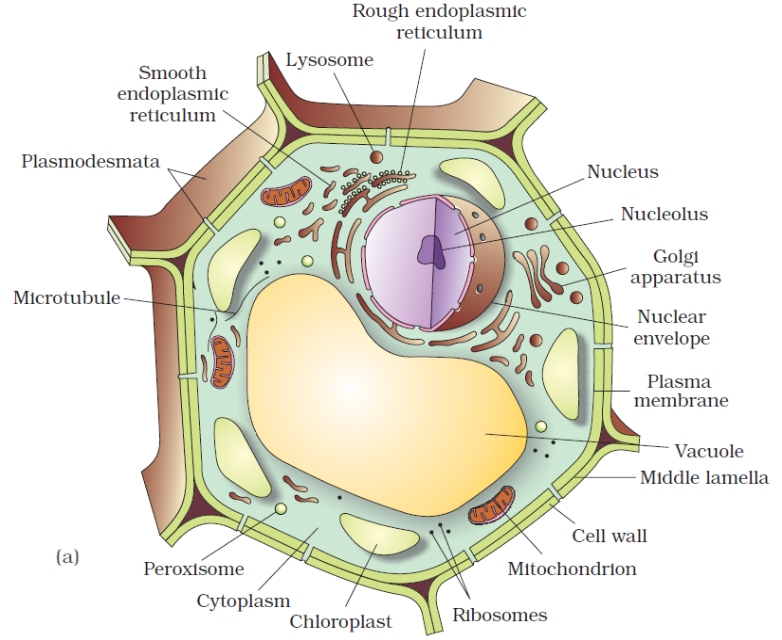
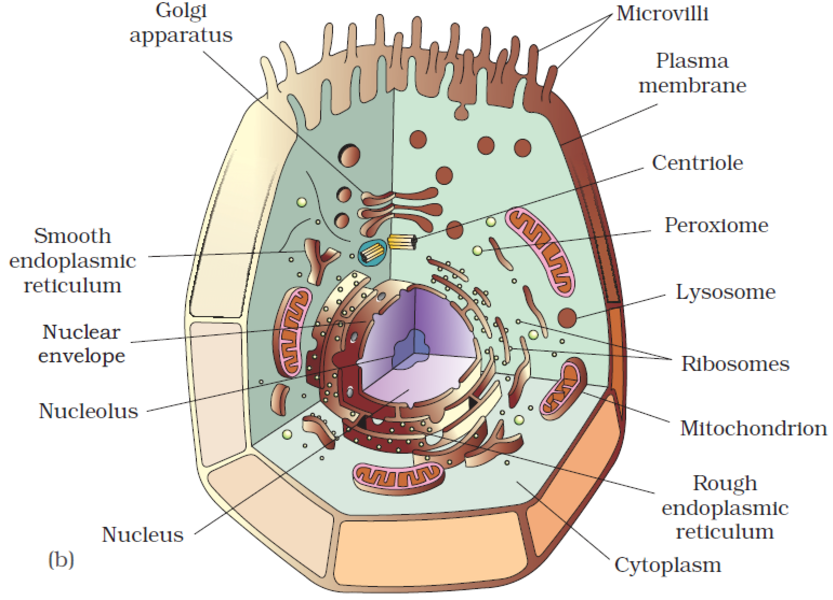
An overview of cell
An overview of the cell
If we observe an onion peel or human cheek cells under a microscope, we will find that the cells in an onion peel show a distinct cell wall as its outermost boundary. The cell membrane lies just inwards to the cell wall. On the other hand, in a human cheek cell, the outermost delimiting structure is the cell membrane. In both, plant and animal cells, a centrally located dense membrane-bound structure called the nucleus is present. This nucleus is known to contain hereditary material i.e. DNA.
Cells that contain a membrane-bound nucleus are known as Eukaryotic cells, whereas the cells in which the nucleus is not defined by a membrane are called Prokaryotic cells. In both prokaryotic as well as eukaryotic cells, the volume of the cell is composed of a semi-fluid matrix called the Cytoplasm. This cytoplasm is the primary site of cellular activities which are important for a living cell.
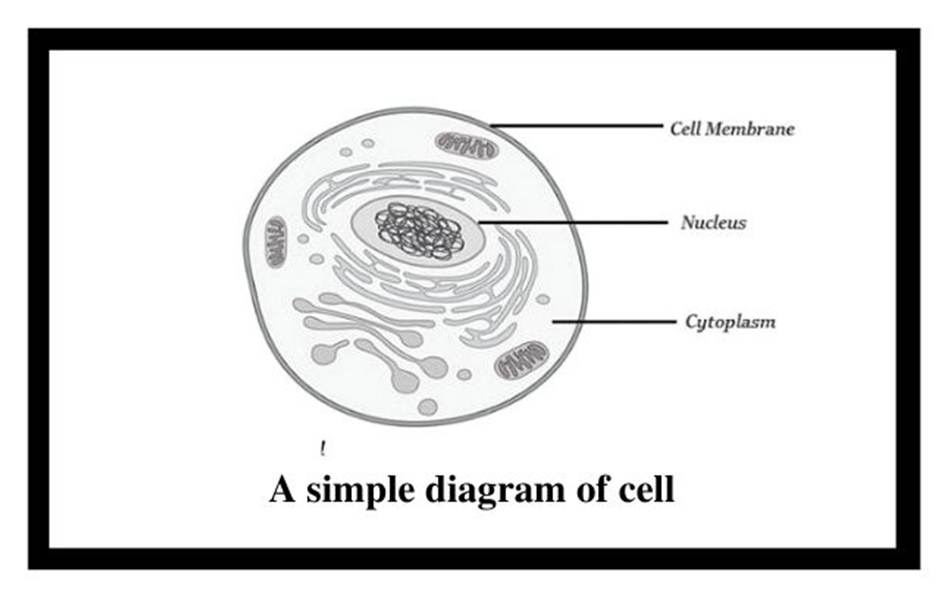
Figure 1: Basic structure of a cell.
Along with the nucleus, a eukaryotic cell also contains cell organelles which are distinct membrane-bound structures. These organelles are responsible for carrying out different metabolic functions. These cell organelles are absent in prokaryotic cells. Some important organelles of a cell are:
a) Mitochondria: are responsible for generating the majority of the chemical energy required by the cell's metabolic activities.
b) Endoplasmic Reticulum: is responsible for protein synthesis.
c) Golgi complex: aids in the processing and packaging of proteins and lipid molecules, particularly proteins destined for cell export.
d) Lysosomes: act as the digestive system of a cell.
e) Vacuoles: help in the storage and disposal of various waste substances in a cell.
f) Ribosomes: are the site for protein synthesis in the cell. They are not bound by a membrane.
g) Centrosome: is also not bound by a membrane. It is present in animal cells and helps in cell division.
Cells can be differentiated based on size, shape, and activities. The smallest cell i.e. Mycoplasma is only 0.3µm long and is even smaller than bacteria. On the other hand, an Ostrich egg, the largest known single cell, is about 15cm to 18 cm long and wide. Cells can be of varying shapes such asdisc-shaped (RBCs), columnar (Goblet cells in the intestine), polygonal (Hydra), cuboid (kidneys), branched (Neuron), elongated (tracheid),and even irregular (Amoeba and WBCs).
An overview of cell
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
AN OVERVIEW OF THE CELL
A typical cell possesses three major elements — outer envelope, genetic material and cytoplasm.
Outer Envelope: A cell is surrounded by an outer membrane called plasma membrane or plasmalemma. It isolates the cell interior. A distinct cell wall lies on its outer side in plant cells. Cell wall provides protection, rigidity and shape to cells.
Genetic Material: It represents hereditary material that not only controls the functioning of the cell but also contains information for forming the whole organism. Genetic material is DNA. In eukaryotes it is enclosed inside the nucleus as chromatin material. The latter appears as chromosomes during cell division. In prokaryotes, the genetic material lies freely inside the cytoplasm as coiled structure called nucleoid.
Cytoplasm: It is semifluid matrix that occupies the interior of cell between nuclear region and outer envelope. Cytoplasm is the area of major cellular or life activities which keep the cell in living state. Certain functions are associated with special cytoplasmic structures called organelles. Organelles are of three types (i) Membrane less, e.g., ribosomes, centrioles, (ii) Single Membranous, e.g., endoplasmic reticulum, Golgi complex, lysosomes, microbodies, sphaerosomes. (iii) Double Membranous, e.g., mitochondria, plastids (in plant cells).
Size and Shape
Cells differ greatly in size, shape and activities. For example, Mycoplasma, the smallest cell, are only 0.3μm in length while bacteria could be 3 to 5μm. The largest isolated single cell is the egg of an ostrich, Acetabularia, a unicellular green alga is about 10 cm in length.
Cell of alga Caulerpa may be upto one metre. Among multicellular organisms, human red blood cells are about 7.0 μm in diameter, nerve fi bres are the longest, upto 90 cm to few metres.
The upper limit or cell size or cell volume is determined by number of factors like :
(i) Metabolic Activity : Metabolically active cells are small in size while less active ones are large, e.g., sperm (active) and egg (passive).
(ii) Nucleocytoplasmic Ratio: Nucleus controls the metabolic activities of the cytoplasm. A higher nucleocytoplasmic ratio provides more efficient metabolic working. (iii) Surface
Volume Ratio : Active cells possess a higher surface : volume ratio. This occurs in small cells, elongated cells and cells with surface invaginations or ingrowths like microvilli of absorptive cells.
Cells also vary greatly in their shape.
They may be disc-like, polygonal, columnar, cuboid, thread like, or even irregular.
The shape of the cell may vary with the function they perform. e.g., RBCs are biconcave to pass through capillaries and carry O2; WBCs are irregular to perform phagocytosis, nerve cells are long to conduct impulses, sperms have tail for motility etc.
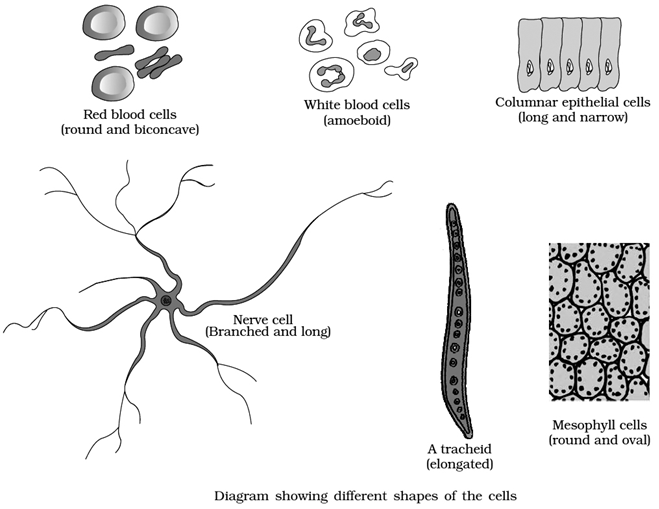
Types of cells
There are two basic types of cells i.e., prokaryotic cells and eukaryotic cells. They are differentiated based on organisation of bio membranes, variety of cytoplasmic organelle and complexity of nuclear material.
Prokaryotic Cells
Prokaryotic cells
The best-known examples of prokaryotic cells are bacteria, blue-green algae, PPLO, and Mycoplasma. Prokaryotic cells multiply more rapidly than eukaryotic cells and are also smaller in size. Their basic shapes are rod-like (bacillus), comma-shaped (vibrio), spherical (coccus), and spiral (spirillum). Despite exhibiting great diversity in shapes, their basic organization remains similar. Except for Mycoplasma, all prokaryotes have a cell wall surrounding their cell membrane. The cytoplasm contains a non-membrane-bound nucleus, containing naked genetic material. Apart from genomic DNA additional circular DNA is also present in prokaryotes known as Plasmid DNA. This plasmid DNA is responsible for imparting unique phenotypes to bacteria. The plasmid DNA also offers antibiotic resistance to the bacterium, which is helpful in case of bacterial transformation. Except for Ribosomes, no other organelle is seen in prokaryotes.
A prokaryotic cell envelope is composed of a tightly bound triple-layered structure. The outermost part of this triple layer is the glycocalyx followed by a cell wall and then the plasma membrane. Each layer has a specific function. Based on the distinctions in the cell envelopes, bacteria are classified into 2 groups namely Gram-positive and Gram-negative. Due to the differences in cell envelops, the bacteria which respond to the Gram stain are regarded as positive whereas the ones that do not take up the stain, are known as Gram-negative.
The composition of glycocalyx differs among different bacterial groups. In some groups, it appears as a loose slimy layer while in others it is present as a thick and sturdy capsule. The cell wall maintains the shape of the cell and provides resistance to the bacterium against disintegration or bursting. The plasma membrane establishes the connection of cells with the outside world. It is selectively permeable in both prokaryotes and eukaryotes.
A unique characteristic of Prokaryotes is the presence of Mesosomes, which are the infoldings of the plasma membrane into the cell. These infoldings can be tubular, lamellar, or vesicular in appearance. They are responsible for cell wall formation, DNA replication, respiration, and secretion processes. In cyanobacteria, other cell membrane modifications are seen which extend into the cytoplasm. They contain pigments and are known as Chromatophores.
Motile bacterial cells possess thin filamentous extensions arising from the cell wall, known as Flagella. A single flagellum consists of a filament, a hook, and a basal body. Apart from flagella, Pili and Fimbriae are also found on the bacterial surface although they have no role to play in motility. Pili are elongated structures made up of proteins and Fimbriae are bristle-like structures erupting from the cell surface. They are known to help the bacteria attach to host tissues or surfaces.
The prokaryotic plasma membrane encloses ribosomes. These ribosomes consist of 2 subunits namely 50s (15nm) and 30s (20nm). Together they form the 70s ribosomal subunit. Ribosomes are known as the site of protein synthesis. They often attach themselves to a single mRNA and form a chain called polyribosome which eventually translates mRNA into proteins.
Prokaryotic cells store their unused or reserved material in specialized non-membranous structures called inclusion bodies which are present in the cytoplasm. Examples of inclusion bodies include glycogen granules, phosphate granules, and cyanophycean granules. In blue-green and purple photosynthetic bacteria, gas vacuoles are also present.
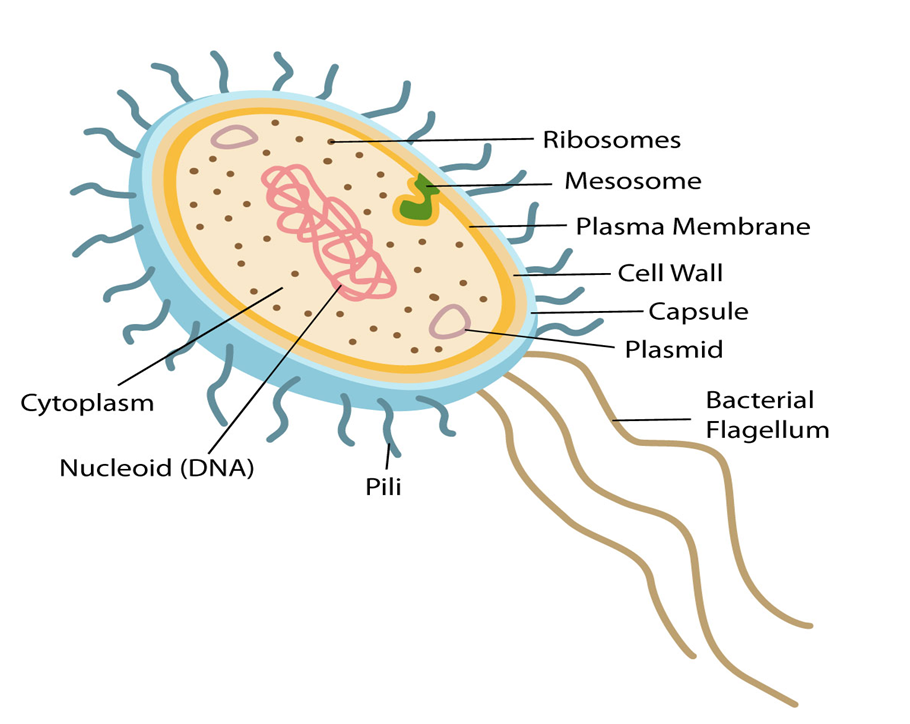
Figure 2: Features of a Prokaryotic cell.
Prokaryotic Cells
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Prokaryotic Cells
1. Cell wall present (bacteria) or absent (mycoplasma)
2. A prokaryotic cell is a single membrane system.
3. Cell membrane bears respiratory enzymes.
4. Mesosomes are formed by infolding of cell membrane.
5. Cytoplasm lacks membrane bound organelles
6. Ribosomes (non membrane bound organelle) are70S, lie free in cytoplasm.
7. There are no streaming movements of cytoplasm.
8. Photosynthetic lamellae i.e., thylakoids (if present) occur free in the cytoplasm.
9. Sap vacuoles are lacking. Gas vacuoles may occur.
10. Transcription and translation occur in the cytoplasm.
11. Protein synthesis takes place in cytoplasm only.
12. Cytoskeleton absent.
13. Nuclear material is not enclosed by nuclear envelope and lies directly in cytoplasm. It is called nucleoid.
14. There is no nucleolus.
15. DNA is closed and circular and without histone core (Polyamines may be present in place of histones)
16. DNA occurs in the cytoplasm only.
17. Plasmids and pili occur in many prokaryotic cells.
18. Flagella, if present, are singlet fibres (9 + 0) and are formed of a protein flagellin.
19. Mitotic spindle is not formed in cell division (Amitotic).
20. Sexual reproduction absent (recombination is present in bacteria)
21. e.g., Bacteria, blue-green algae and mycoplasmas.
Eukaryotic Cells
Eukaryotic Cells
Eukaryotes refer to any cell or organism having an identifiable nucleus. The nucleus of a eukaryotic cell is surrounded by a nuclear membrane, which contains well-defined chromosomes. Plants, animals, protists, and fungi are examples of eukaryotic cells. Chromosomes contain their genetic material. Eukaryotic cells have organelles such as the Golgi apparatus, Mitochondria, Ribosomes, and Nucleus. They have intricate locomotory and cytoskeletal features, as well.
Among eukaryotes, plant and animal cells show structural dissimilarities. Plant cells possess a cell wall, plastids, and a large central vacuole. An animal cell, on the other hand, possesses a centriole that is absent in the plant cell.
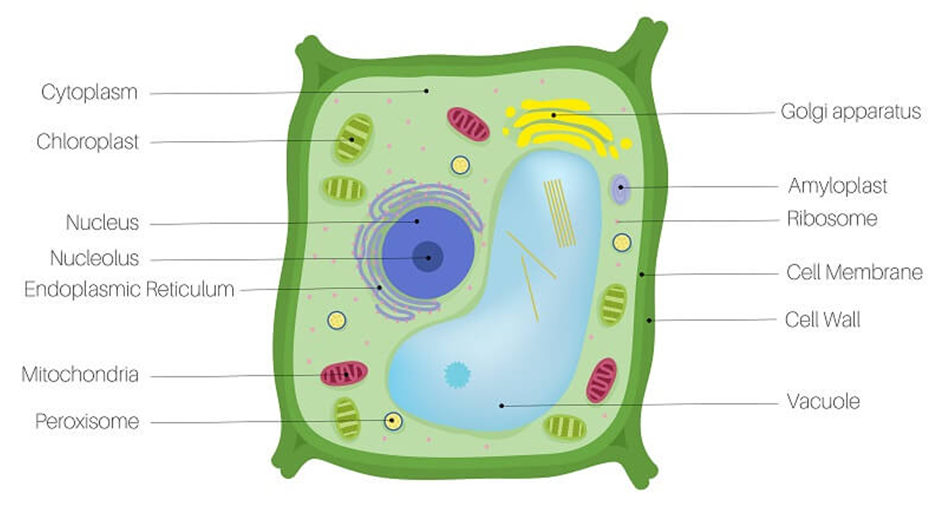
Figure 3: A plant cell.
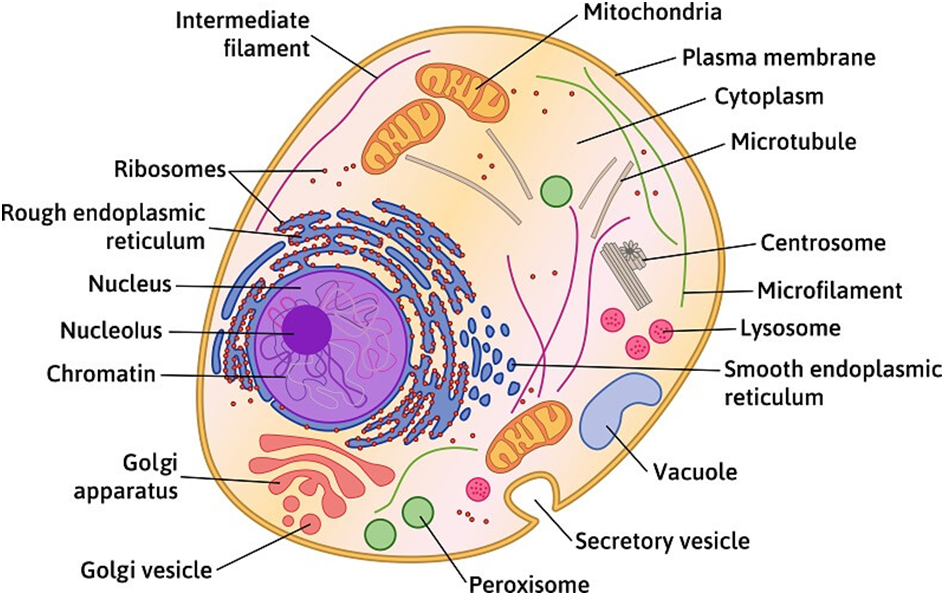
Figure 4: An Animal Cell.
(A)Cell Membrane:
The cell membrane, also known as the plasma membrane, separates the interior of the cell from the outside environment and is found in all cells. A semipermeable lipid bilayer makes up the cell membrane. The transfer of materials into and out of the cell is controlled by the cell membrane. In the late 1600s, Robert Hooke discovered the cell membrane.
Lipids and proteins make up the majority of the cell membrane. Phospholipids, which are organized in a bilayer, are the most important lipids. The lipids are also organized within the membrane with the polar heads on the outside and the hydrophobic tails on the inside, with the polar heads on the outside and the hydrophobic tails on the inside. This guarantees that saturated hydrocarbons' nonpolar tails are protected from the aqueous environment. Membranes include cholesterol in addition to phospholipids.
Biochemical analysis later revealed that cell membranes contain protein and carbohydrates as well. In different cell types, the protein-to-lipid ratio varies greatly. For example, the erythrocyte membrane contains around 52 percent protein and 40 percent lipids in humans.Membrane proteins are divided into two categories: integral and peripheral. Integral proteins are partially or completely buried in the membrane, whereas peripheral proteins are on the surface.
Singer and Nicolson (1972) provided an improved model of cell membrane construction that is now widely accepted as the fluid mosaic model. The quasi-fluid property of lipid, according to this, allows for lateral mobility of proteins within the total bilayer. The fluidity of a membrane is a measure of its capacity for internal movements. The fluid nature of the membrane is also critical for tasks such as cell proliferation, intercellular connection creation, secretion, endocytosis, and cell division.
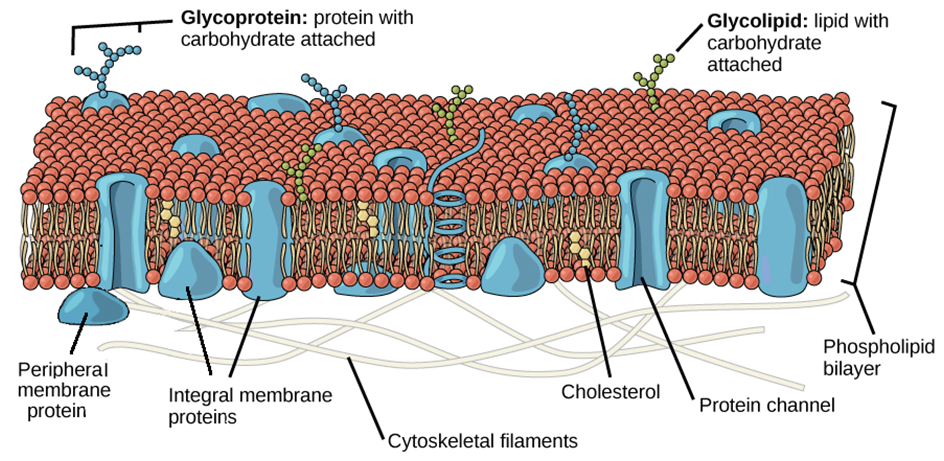
Figure 5: Fluid Mosaic Model of the plasma membrane.
The transport of molecules across the plasma membrane is one of the most significant tasks performed by this structure. Some molecules on each side of the membrane are selectively permeable through the membrane. The term "passive transport" refers to the ability of several molecules to travel across a membrane without requiring any energy. Simple diffusion along a concentration gradient, i.e. from a higher to a lower concentration, can transport neutral solutes across the membrane. Water can also travel from a greater to a lower concentration across this membrane. Osmosis is the movement of water by diffusion. As polar molecules cannot pass through the nonpolar lipid bilayer, they must be transported across the membrane by a membrane carrier protein.A few ions or molecules are transported across the membrane in the opposite direction of their concentration gradient, that is, from lower to higher concentration. Active transport, for example, is an energy-dependent activity that uses ATP as observed in a sodium-potassium pump.
(B) Cell Wall:
The cell wall is a non-living inflexible structure that is only found in plants and fungi. A cell wall not only gives the cell structure and protects it from mechanical damage and infection, but it also aids cell-to-cell communication and acts as a barrier to undesirable macromolecules. Algae have cellulose, galactans, mannans, and minerals like calcium carbonate in their cell walls, whereas other plants have cellulose, hemicellulose, pectins, and proteins. The primary wall of a young plant cell can develop, but as the cell grows, the primary wall shrinks, and the secondary wall forms on the inner (towards membrane) side of the cell.The middle lamella is a calcium pectate-based layer that binds or glues the surrounding cells together. Plasmodesmata, which connect the cytoplasm of neighboring cells, can pass through the cell wall and middle lamellae.
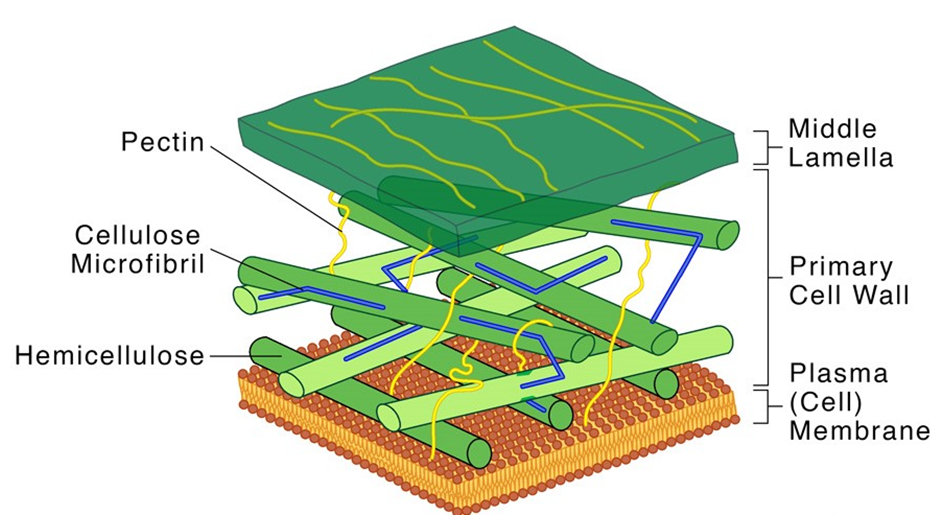
Figure 6: Structure of a Cell Wall.
Each membranous organelle has its structure and function, however, those organelles whose functions are coordinated, are grouped as an endomembrane system. Endoplasmic reticulum (ER), Golgi complex, lysosomes, and vacuoles are all part of the endomembrane system. The mitochondria, chloroplast, and peroxisomes are not considered part of the endomembrane system because their functions are not coordinated with those of the above organelles.
(C) Endoplasmic Reticulum (ER):
Upon observing a eukaryotic cell under an electron microscope, it was discovered that there is a network or reticulum of microscopic tubular structures dispersed throughout the cytoplasm.This network was identified as Endoplasmic Reticulum (ER). It divides the intracellular cavity into 2 parts namely, luminal (within ER) and extraluminal (cytoplasm). Ribosomes are frequently seen attached to the outer surface of ER. This is known as Rough ER (RER). RER is found in a lot of cells that are involved in protein synthesis and secretion. They are long and contiguous with the nucleus's outer membrane. Smooth ER (SER) refers to the absence of ribosomes on ER surface. The SER is the primary location for lipid synthesis. SER produces lipid-like steroidal hormones in animal cells.
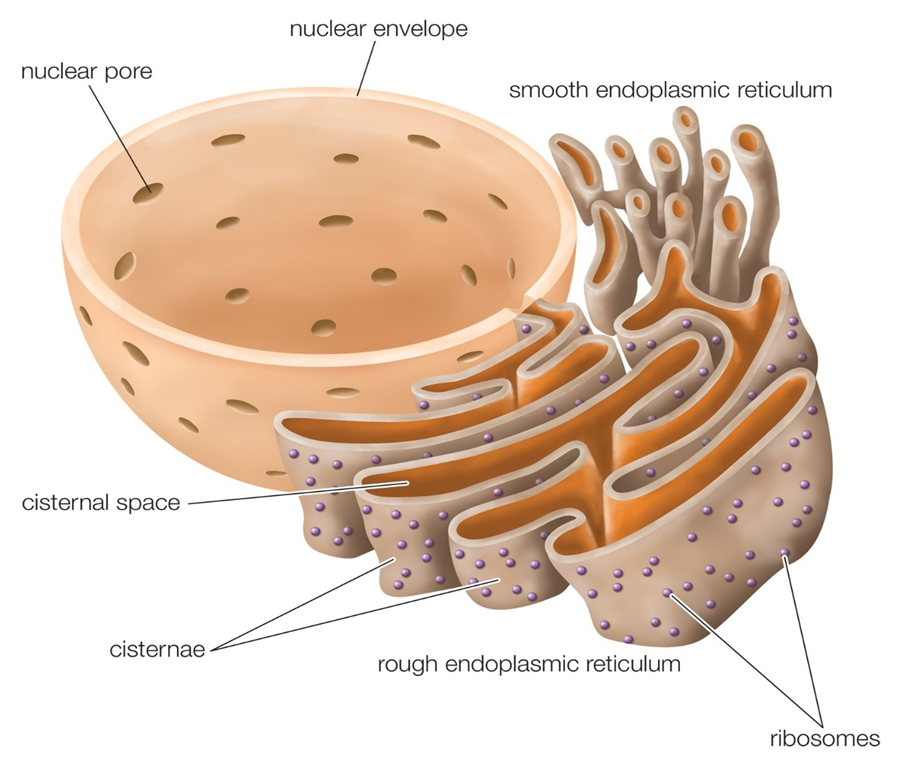
Figure 7: Endoplasmic Reticulum.
(D) Golgi apparatus:
In the year 1898, Camillo Golgi, an Italian researcher, first identified the heavily pigmented reticular structures near the nucleus. These entities were given the name Golgi bodies. They are made up of a large number of flat, disc-shaped sacs or cisternae with a diameter of 0.5m to 1.0m. These are piled one on top of the other. In a Golgi complex, there are a variety of cisternae. The Golgi cisternae are organized in a concentric pattern near the nucleus, with distinct convex Cis (forming face) and concave Trans (maturing face) face. The organelle's Cis and Trans faces are completely different, although they are linked. The Golgiapparatus is primarily responsible for packing materials for delivery to intracellular targets or secretion outside the cell.
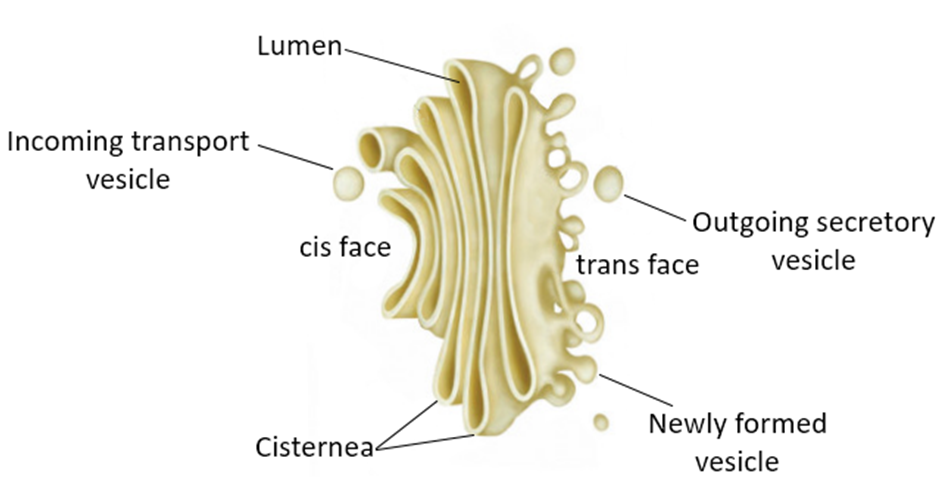
Figure 8: The Golgi apparatus.
(E) Lysosomes:
Lysosomes are membrane-bound vesicular organelles generated during the Golgi apparatus packaging process. Almost all types of hydrolytic enzymes (hydrolases – lipases, proteases, carbohydrases) were observed to be abundant in the isolated lysosomal vesicles, which are best active at acidic pH. Carbohydrates, proteins, lipids, and nucleic acids can all be digested by these enzymes. They are spheres made up of a lipid bilayer that encloses fluid containing a range of hydrolytic enzymes and have a simple structure.
Vacuoles:
In the cytoplasm, the vacuole is a membrane-bound compartment. It contains water, sap, excretory product, and other non-cellular elements. Tonoplast is a single membrane that separates the vacuole from the rest of the cell. Plant cells have vacuoles that can take up to 90% of the cell's volume. The tonoplast in plants accelerates the transport of ions and other materials over concentration gradients into the vacuole, resulting in a substantially higher concentration in the vacuole than in the cytoplasm. The contractile vacuole is crucial for osmoregulation and excretion in Amoeba. Food vacuoles are generated by engulfing food particles in various organisms, including protists.
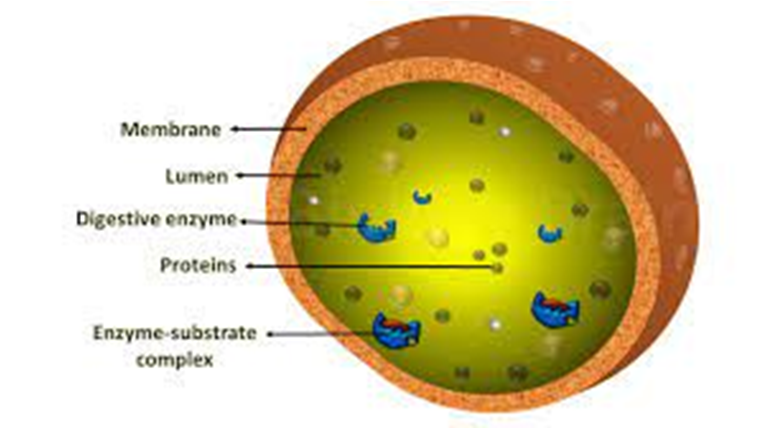
Figure 9: Lysosomes
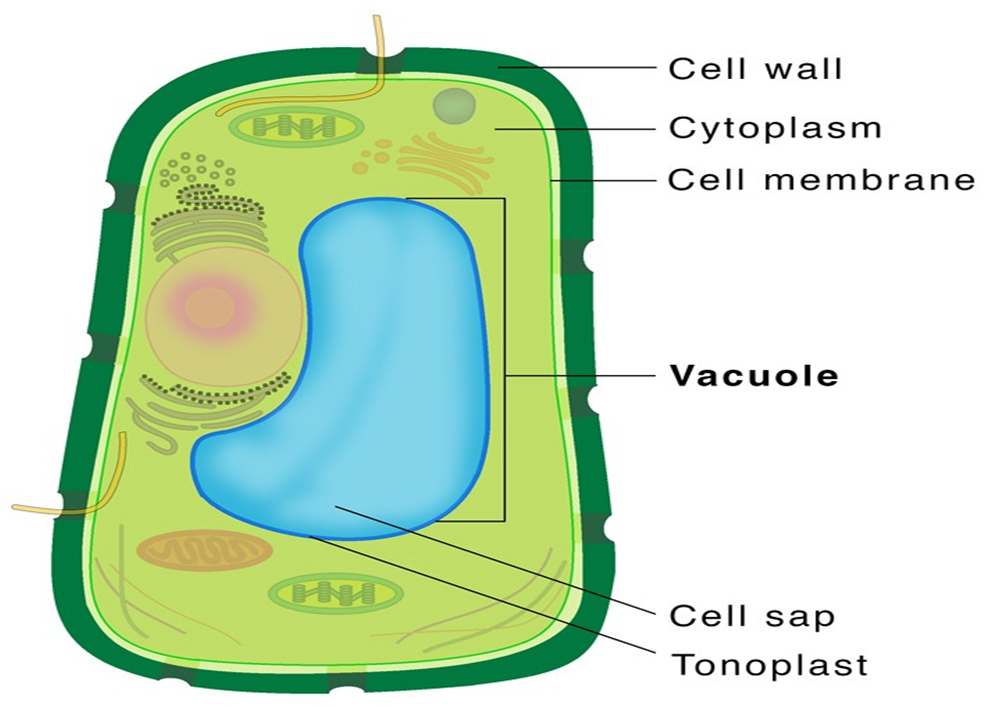
Figure 10: Vacuole.
(F) Mitochondria:
Mitochondria (plural: mitochondrion) are membrane-bound cell organelles that provide the majority of the chemical energy required to fuel the cell's metabolic activities. Adenosine triphosphate (ATP) is a tiny molecule that stores the chemical energy created by mitochondria. Each mitochondrion is a double membrane-bound structure, with the outer and inner membranes partitioning the lumen into two different aqueous compartments, the outer and inner compartments, respectively. The matrix is a solid, homogeneous substance that fills the inner compartment. The organelle's continuous limiting boundary is formed by the outer membrane. Towards the matrix, the inner membrane creates a series of infoldings called cristae (sing.: crista). The surface area is increased by the cristae. The enzymes linked with the two membranes are different.The two membranes each contain their own set of mitochondrial function-related enzymes. Aerobic respiration takes place in mitochondria. They are known as the 'power houses' of the cell because they produce cellular energy in the form of ATP. A single circular DNA molecule, a few RNA molecules, ribosomes (the 70S), and the components required for protein synthesis are also present in the mitochondrial matrix. Fission is the process through which mitochondria divide.
Mitochondria are difficult to see under a microscope unless they are stained specifically. The quantity of mitochondria per cell varies according to the cells' physiological activity. There is a great deal of variation in terms of shape and size as well.
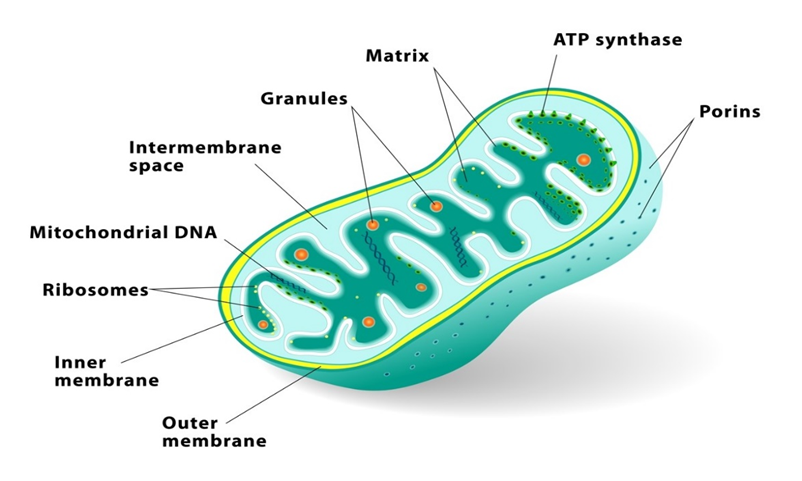
Figure 11: Mitochondria.
(G) Plastids:
All plant cells and euglenoids contain plasmids. Because they are big, they are easily visible under a microscope. They contain unique pigments, which give the plants their colors Plastids are divided into three types based on the pigments they contain: chloroplasts, chromoplasts, and leucoplasts. Chlorophyll and carotenoid pigments are found in chloroplasts, and they are important for capturing the light energy required for photosynthesis. Carotene, xanthophylls, and other fat-soluble carotenoid pigments are found in the chromoplasts. The plant's portion turns yellow, orange, or red as a result of this. Amyloplasts store carbohydrates (starch) inpotatoes, whereas elaioplasts store oils and lipids, and aleuroplasts store proteins.
The mesophyll cells of the leaves contain the majority of the chloroplasts in green plants. These organelles can be lens-shaped, oval, spherical, discoid, or even ribbon-like, with varying lengths (5-10m) and widths (2-4m). Their numbers range from one per cell in the green alga Chlamydomonas to 20-40 per cell in the mesophyll.
The chloroplasts, like mitochondria, a membrane-boundary bound. The inner chloroplast membrane is the less permeable of the two. The stroma is the compartment enclosed by the chloroplast's inner membrane. The stroma contains several thylakoids, which are flattened membrane sacs that are structured. Thylakoids are placed in stacks similar to grana (singular: granum) or intergranal thylakoids, which are coin piles. In addition, the stroma lamellae are flat membranous tubules that connect the thylakoids of the various grana. Thylakoids have a lumen that is enclosed by their membrane. The thylakoids contain chlorophyll pigments.Enzymes necessary for glucose and protein synthesis are found in the stroma of chloroplasts. It also contains ribosomes and tiny double-stranded circular DNA molecules.The chloroplast ribosomes (the 70S) are smaller than the cytoplasmic ribosomes (80S).
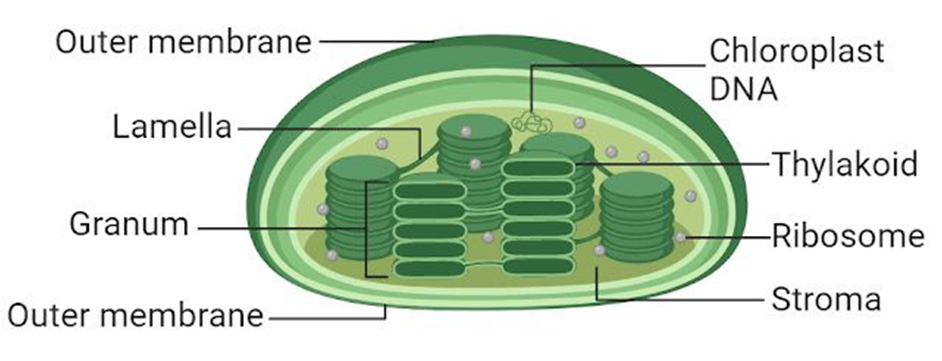
Figure 12: Plastids
(H) Ribosomes:
Ribosomes are granular structures that were first discovered as dense particles by George Palade (1953) through electron microscopy. They are made up of ribonucleic acid (RNA) and proteins and do not have a membrane surrounding them. The ribosomes of eukaryotes are, while those of prokaryotes are the 70S. There are two subunits in each ribosome: bigger and smaller subunits (Fig 8.9). The 60S and 40S are the two subunits of 80S ribosomes, while 50S and 30S are the subunits of 70S ribosomes.
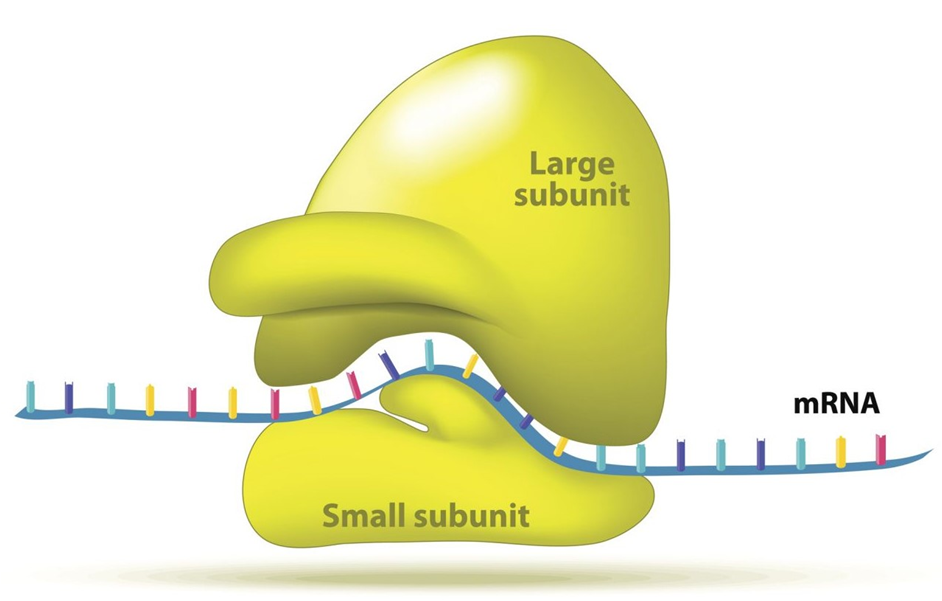
Figure 13: A Ribosome.
(I) Cytoskeleton:
The cytoskeleton is an intricate network of filamentous proteinaceous structures found in the cytoplasm that includes microtubules, microfilaments, and intermediate filaments. The cytoskeleton in a cell is engaged in a variety of tasks, including mechanical support, motility, and cell shape maintenance.
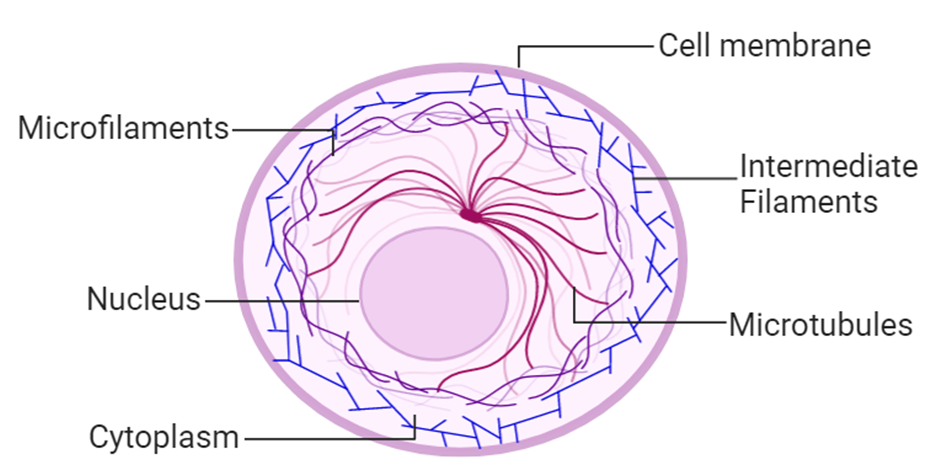
Figure 14: Cytoskeleton
(J) Cilia and Flagella:
Cilia (singular: cilium) and flagella (singular: flagellum) are hair-like protrusions from the cell membrane. Cilia are tiny structures that act like oars, causing the cell or the surrounding fluid to move. Flagella, on the other hand, are longer and are in charge of cell motility. Flagella are also seen in prokaryotic bacteria, however, they differ structurally from eukaryotic flagella.
An electron microscope examination of a cilium or flagellum reveals that they are encased in a plasma membrane. The axoneme, or core, severalseveralrotubules that run parallel to the long axis. Nine doublets of radially oriented peripheral microtubules and a pair of centrally positioned microtubules make up the axoneme. The 9+2 array refers to a specific arrangement of axonemal microtubules.
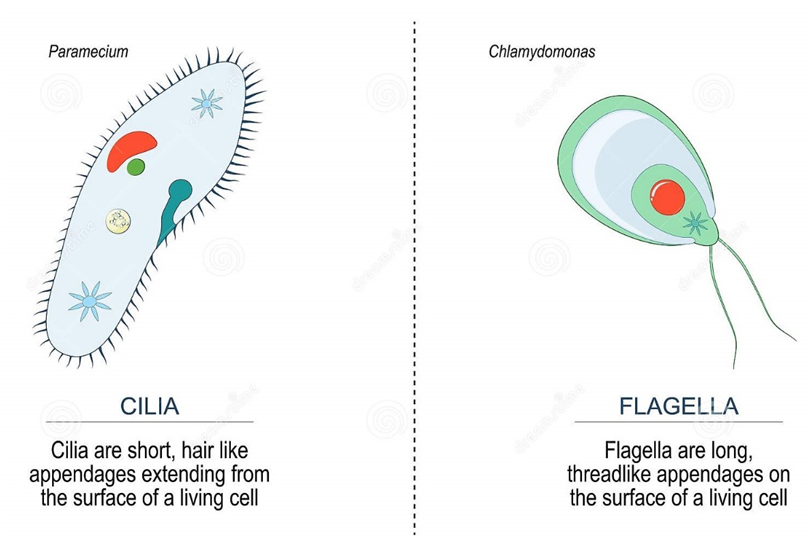
Figure 15: Difference between Cilia and Flagella.
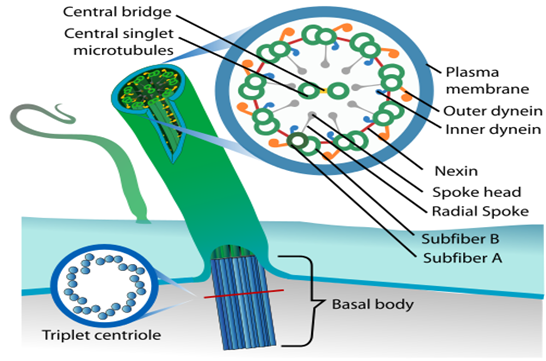
Figure 16: Structural arrangement in Cilia and Fagella.
The central tubules are linked by bridges and encased by a central sheath, which is linked to one of the tubules of each peripheral doublet by a radial spoke. There are nine radial spokes as a result. Linkers are also used to connect the peripheral doublets. The cilium and flagellum both emerge from the basal bodies, which are centriole-like structures.
(K) Centrosome and Centrioles:
A centrosome is an organelle that normally has two centrioles, which are cylindrical structures. Pericentriolar materials, which are amorphous, surround them. Both centrioles in a centrosome are perpendicular to one other and have a cartwheel-like arrangement. They are made up of nine tubulin protein peripheral fibrils that are uniformly spaced. Each peripheral fibril is made up of three triplets. The triplets next to it are also related. The hub, which is connected to tubules of the peripheral triplets by radial spokes comprised of protein, is located in the center section of the proximal region of the centriole. During cell division in animal cells, the centrioles create the basal body of cilia or flagella, as well as spindle fibers that give rise to the spindle apparatus.
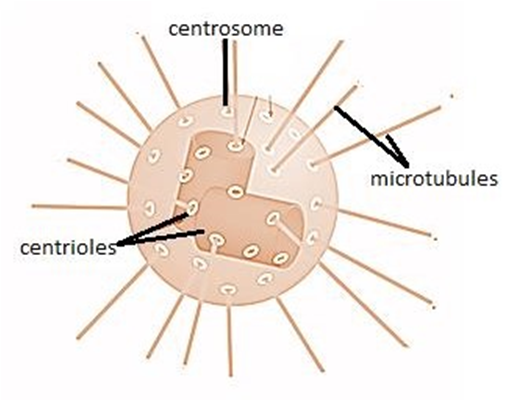
Figure 17: A centrosome and a centriole.
(L) Nucleus:
A nucleus is a membrane-bound organelle that governs and regulates the cell's functions (such as development and metabolism) and houses the structures that contain hereditary information, such as genes. Robert Brown was the first to characterize the nucleus in 1831. The nucleus is made up of a nuclear envelope, which is made up of two parallel membranes with a space between them termed the perinuclear space, according to electron microscopy. The nuclear membrane serves as a barrier between the contents of the nucleus and the cytoplasm. The outer membrane is normally connected to the endoplasmic reticulum and contains ribosomes. The nuclear envelope is punctured at several points by minute pores created by the merging of its two membranes.
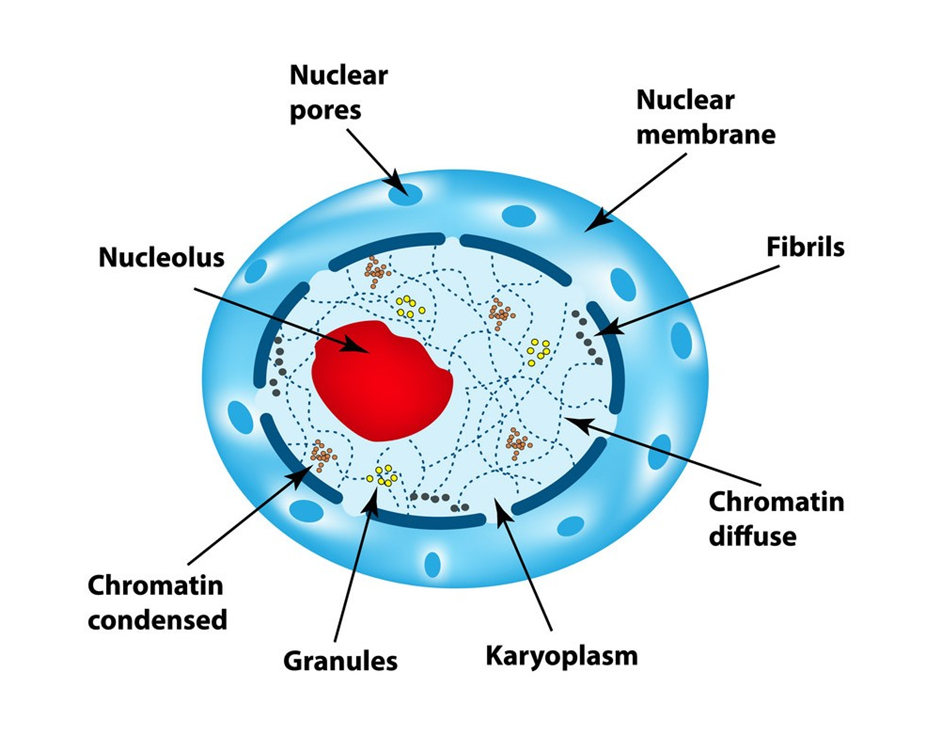
Figure 18: Nucleus
These nuclear pores are the conduits through which RNA and protein molecules travel between the nucleus and the cytoplasm in both directions. Although each cell normally has only one nucleus, variations in the number of nuclei are common. Nucleolus and chromatin are found in the nuclear matrix, also known as nucleoplasm. In the nucleoplasm, there are spherical structures called nucleoli. Because the nucleolus is not a membrane-bound structure, its contents are continuous with the rest of the nucleoplasm. It's where active ribosomal RNA synthesis happens. In cells that are actively synthesizing proteins, nucleoli are larger and more numerous.
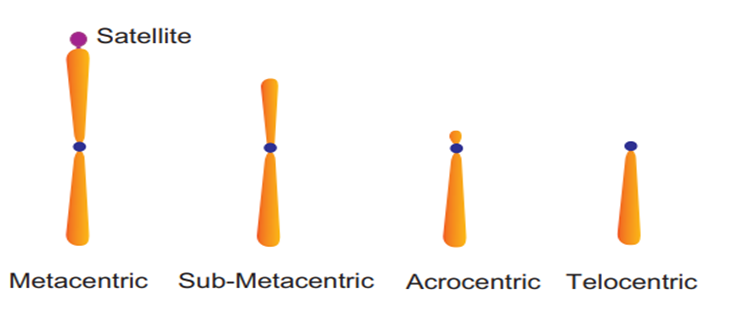
Figure 19: Types of Chromosomes.
The interphase nucleus is chromatin, which is a loose and unclear network of nucleoprotein fibers. Cells, on the other hand, reveal organized chromosomes in place of the nucleus at various phases of cell division. DNA, histones (basic proteins), non-histone proteins, and RNA are all found in chromatin. Each of the forty-six (twenty-three pairs) chromosomes in a single human cell has a two-meter-long strand of DNA.
Every chromosome (visible only in dividing cells) has a central constriction called the centromere, which is flanked by disc-shaped structures called kinetochores. A chromosome's centromere holds two chromatids.The chromosomes can be divided into four categories based on the position of the centromere. The central centromere of the metacentric chromosome divides the chromosome into two equal arms. The centromere of the sub-metacentric chromosome is located somewhat distant from the chromosome's center resulting in one shorter and one long arm. The centromere of an acrocentric chromosome is at the end, forming one extremely short and one extremely long arm, whereas a telocentric chromosome contains a terminal centromere.Occasionally, a few chromosomes will have non-staining secondary constrictions at the same spot. This appears to be a little component known as the satellite.
Microbodies:
Microbodies are membrane-bound minute vesicles that contain a variety of enzymes. Both plant and animal cells have them. Peroxisomes, glyoxysomes, glycosomes, and hydrogenosomes are all microbody organelles. Microbodies are particularly common in the liver and kidney of vertebrates.
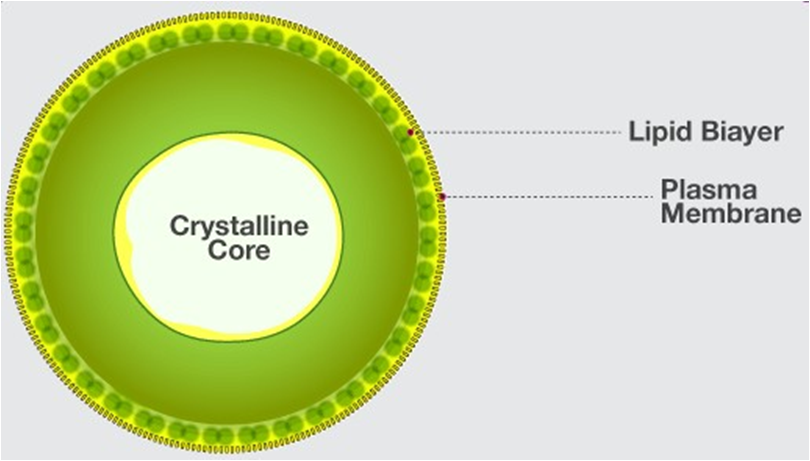
Figure 20.
Eukaryotic Cells
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Eukaryotic cells
1. Cellulosic cell wall (Plants) or absent (Animals)
2. A eukaryotic cell is a double membrane system.
3. Cell membrane lacks respiratory enzymes.
4. Mesosomes are absent.
5. Cytoplasm contains membrane bound organelles, (endoplasmic reticulum, mitochondria, golgi apparatus, lysosomes and centrosome.
6. Ribosomes are 80 S, may lie free or bound to E.R. and nuclear envelope. (70 S ribosomes are found within mitochondria and chloroplast)
7. Cytoplasm shows streaming movements (cyclosis).
8. Photosynthetic lamellae if present, occur within
9. the chloroplasts.
10. Sap vacuoles are common.
11. Transcription and translation occur in nucleus and cytoplasm respectively.
12. Protein synthesis occurs in the cytoplasm,
13. mitochondria and plastids.
14. Cytoskeleton (microtubule, microfilament and
15. intermediate filaments) present.
16. Nuclear material is enclosed by nuclear envelope to form a nucleus distinct from cytoplasm.
17. One or more nucleoli occur within the nucleus.
18. Nuclear DNA is linear with a histone protein core.
19. DNA occurs in the nucleus as well as in mitochondria and chloroplasts.
20. There are no plasmids and pili in eukaryotic cells.
21. Flagella, if present, are complex, have 9 + 2 pattern of microtubules formed of a protein tubulin.
22.Mitotic spindle is formed in cell division.
23. Sexual reproduction occurs.
e.g., Algae other than blue-green algae, protists, fungi, plants and animals.
How to Analyse Chemical Composition?
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Protoplasm is a complex mixture of both organic and inorganic compounds.
Molecules found in the protoplasm of cells are called biomolecules.
A Comparison of Elements Present in Non-living and Living matter
The collection of various types of molecules in a cell is called the cellular pool.
The cellular pool consists of various types of biomolecules such as : (a) water (b) inorganic materials (c) organic compounds.
The small molecules of low molecular weight, simple molecular conformations and higher solubilities are called micromolecules.
These include minerals, water, amino acids, simple sugars and nucleotides.
The various minerals found in cells have many uses.
Mitochondria are rich in manganese.
Molybdenum is necessary for fixation of nitrogen catalysed by the enzyme nitrogenase.
Copper occurs in cytochrome oxidase.
Magnesium is essential for a large number of enzymes, particularly those utilising ATP.
Ca and Mg decrease the excitability of nerves and muscles.
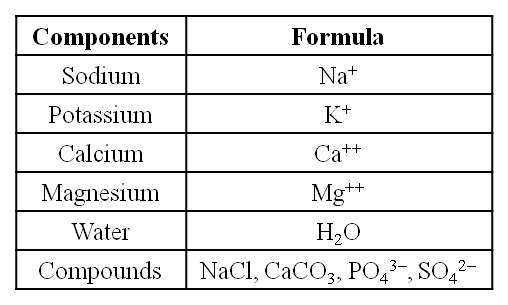
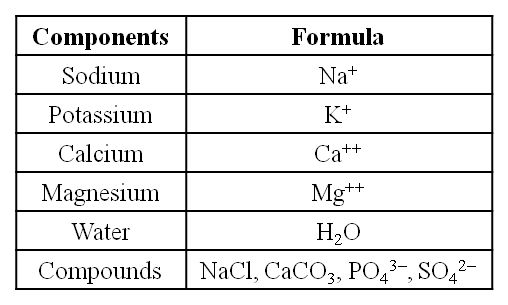
(i) Sodium and potassium are responsible for the maintenance of extracellular and intracellular fluids through the osmotic effects of their concentration. These two ions are also responsible for the maintenance of membrane potential and transmission of electrical impulses in the nerve cells. Both in cells and in extracellular fluids, diabasic phosphate (HPO42–) and monobasic phosphate (H2PO42–) act as acidbase buffers to maintain the H+ ion concentration.
(ii) The most abundant element in cell/living matter is oxygen. O > C > N > H
(iii) Fe++ and Cu++ are found in cytochromes.
(iv) The concentration of the cations inside the cell is K > Na > Ca.
How To Analyse Chemical Composition?
In order to study the various biomolecules found in living tissues (a vegetable or a piece of liver etc.), the tissue is ground in trichloroacetic acid (Cl3CCOOH) using pestle and mortar.
The resultant slurry is strained through cheese cloth or cotton and we obtain two fractions.
The filtrate is called acid soluble pool while the retentate is called acid insoluble fraction.
The acid soluble pool represents roughly the cytoplasmic composition.
The macro molecules from cytoplasm and organelles become the acid-insoluble fraction.
Chemicals present in both the fractions are further separated by various analytical techniques and identified.
Average Composition of Cells
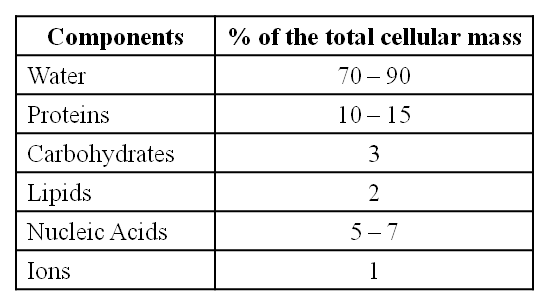
Note : Protein > Nucleic acid > Carbohydrates > Lipids
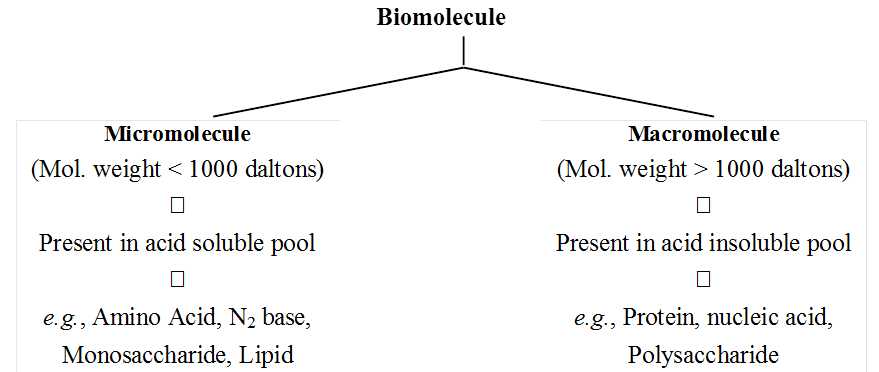
The acid soluble pool contains chemicals called biomicromolecules as they have small molecular mass of 18-800 daltons approximately.
The acid insoluble fraction contains chemicals with large molecular mass of more than 800 daltons, they are biomacromolecules.
Biomacromolcules are large size, high molecular weight, complex molecules that are formed by condensation of biomicromolecules.
Their molecular mass is in the range of ten thousand daltons and above.
Biomacromolecules are of three types-proteins, nucleic acids and polysaccharides.
Note : Though lipids have a molecular mass similar to that of micromolecules i.e. less than 800 Da, but they do not appear in the acid Soluble pool due to their non-polar nature.
All biomacromolecules are polymers except lipids.
Polymers are formed by process of union of repeating subunits, each subunit being called monomer.
Monomers are simple small sized low molecular weight molecules which cannot be hydrolysed further into smaller subunits.
Polymers occur in the form of threads.
They are folded variously to form three-dimensional shapes required for their functioning.
A list of Representative Inorganic Constituents of Living Tissues
Depending upon the molecular weight and solubility, biomolecules are divided into two categories.
(a) Micromolecules are small sized, have low molecular weight, simple molecular structure and high solubility in the intracellular fluid matrix. These include water, mineralrs, gases, carbohydrates, lipids, amino acids and nucleotides.
(b) Macromolecules are large sized, have larger molecular weight, complex conformation and low solubility in the intracellular fluid matrix. They are generally formed by polymerisation of micromolecules. These include polysaccharides, proteins and nucleic acids.
Analytical techniques, when applied to the compound give us an idea of the molecular formula and the probable structure of the compound.
All the carbon compounds that we get from living tissue can be called "Biomolecules".
However, living organisms also have inorganic elements and compounds.
When the tissue is fully burnt all carbon compounds are oxidised to gaseous form (CO2, water vapour) and are removed.
What is remaining is called "Ash".
This ash contains inorganic elements (like calcium, magnesium etc.).
Inorganic compounds like sulphate, phosphate etc. are also seen in the acid soluble fraction.
Therefore, elemental analysis gives elemental composition of living tissues in the form of H, O, Cl, C etc. while analysis of compounds gives an idea of the kind of organic and inorganic constituents present in living tissues.
From a chemistry point of view, one can identify functional groups like aldehydes, ketones, aromatic compounds etc.
But from a biological point of view, we shall classify them into aminoacids, nucleotide bases, fatty acids etc.
How to Analyse Chemical Composition?
Chapter 9
Biomolecules
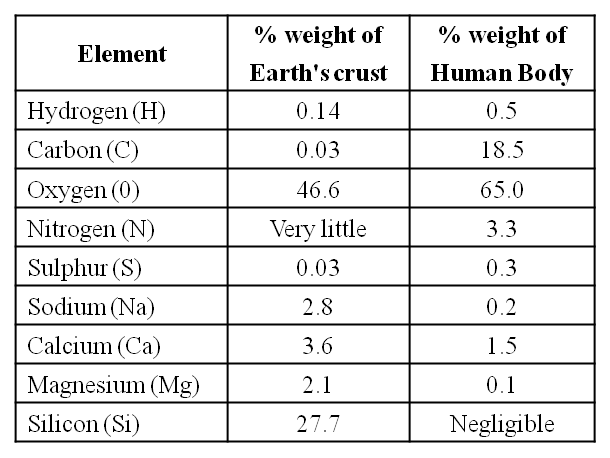
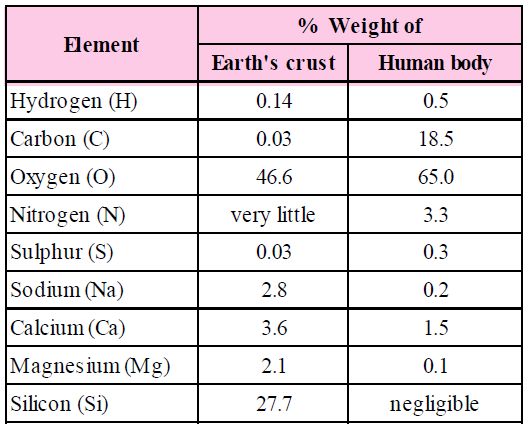
The biosphere has a vast variety of living species. Carbon, hydrogen, oxygen, and numerous other elements, as well as their respective contents, are acquired per unit mass of living tissue when an elemental analysis is done on plant tissue, animal tissue, or microbial paste. A similar list of elements will be obtained if the same analysis is performed on a piece of the earth's crust as an example of non-living stuff. A sample of live tissue contains all of the elements found in a sample of the earth's crust. However, a closer look reveals that any living organism has a higher relative quantity of carbon and hydrogen in relation to other elements than the earth's crust.
How to Analyse Chemical Composition?
A natural substance's elemental analysis reveals that it is made up of several elements such as carbon, hydrogen, oxygen, chlorine, and so on. Analytical procedures provide information on various organic and inorganic compounds, as well as their molecular formulas and structures. They also aid in the separation and purification of one component from another. Simple experiments can be used to determine the chemical composition of biomolecules. Crush and combine a piece of living tissue with an acid. We get two pieces after filtering it. The acid-soluble fraction of the filtrate is retained on the filter membrane, while the acid-insoluble fraction is retained on the filter membrane. This indicates that there are two or more substances with distinct characteristics within the tissues.Thousands of organic molecules have been discovered in the acid-soluble pool by scientists.
Constituents of Living Tissues
Take another piece and burn it till all the moisture in it is evaporated. When carbon compounds are burned, they are all oxidized. Inorganic substances such as calcium, magnesium, sulfate, phosphate, and others are formed in the tissue by the ash that has been left out.
Analytical techniques, when applied to a chemical, provide us with an estimate of its molecular formula and likely structure. 'Biomolecules' refers to all carbon compounds obtained from living tissues. All carbon-containing chemicals (organic compounds) found in living organisms are classified as biomolecules. They are organic substances found in living cells that have a role in the organism's maintenance and metabolic activities. Inorganic elements and compounds, on the other hand, are found in living beings. As a result, elemental analysis provides information on the composition of live tissues in terms of hydrogen, oxygen, chlorine, carbon, and other elements, whereas compound analysis provides information on the types of organic and inorganic constituents found in living tissues.
Functional groups such as aldehydes, ketones, aromatic molecules, and others can be detected in living tissues from a chemical standpoint. However, amino acids, nucleotide bases, fatty acids, and carbohydrates make up living tissues from a biological standpoint.
Amino acids are chemical molecules that have both an amino and an acidic group as substituents on the same carbon, the -carbon. As a result, they are known as -amino acids. They're methanes that have been replaced. The four valency locations are occupied by four substituent groups. Hydrogen, carboxyl group, amino group, and a variable group known as R group are the four groups. There are numerous amino acids in the R group due to its nature. However, there are only twenty varieties of those found in proteins. The R group in these proteinaceous amino acids could be a hydrogen (glycine), a methyl group (alanine), hydroxy methyl (serine), or something else entirely. The amino, carboxyl, and R functional groups make up the chemical and physical properties of amino acids.Acidic (e.g., glutamic acid), basic (lysine), and neutral (valine) amino acids are classified by the number of amino and carboxyl groups they contain.
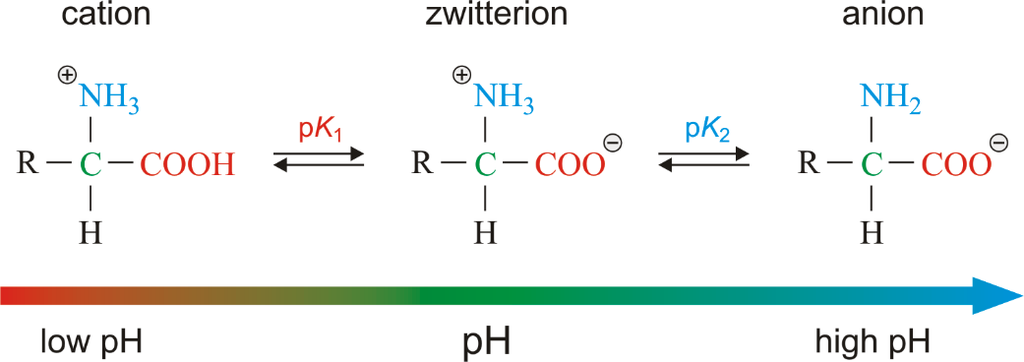
Similarly, aromatic amino acids exist (tyrosine, phenylalanine, tryptophan). The ionizability of the –NH2 and –COOH groups of amino acids is a unique feature. As a result, the structure of amino acids alters in different pH solutions.
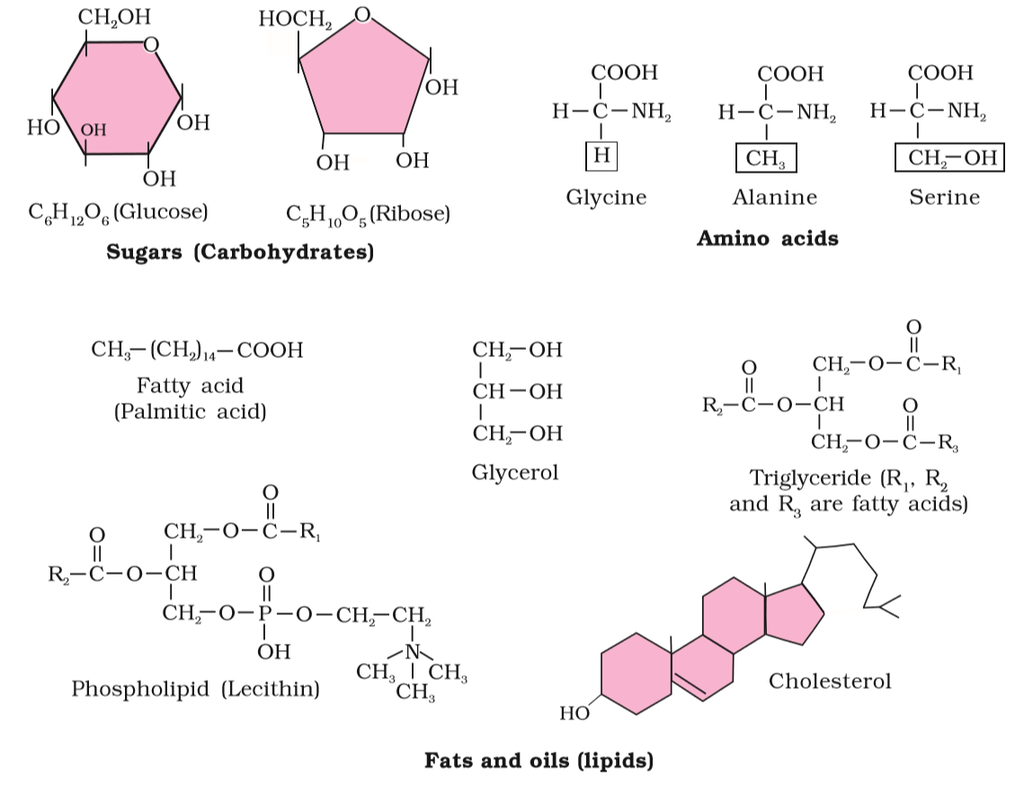
Figure 2 (a): Diagrammatic representation of small molecular weight organic compounds in living tissues
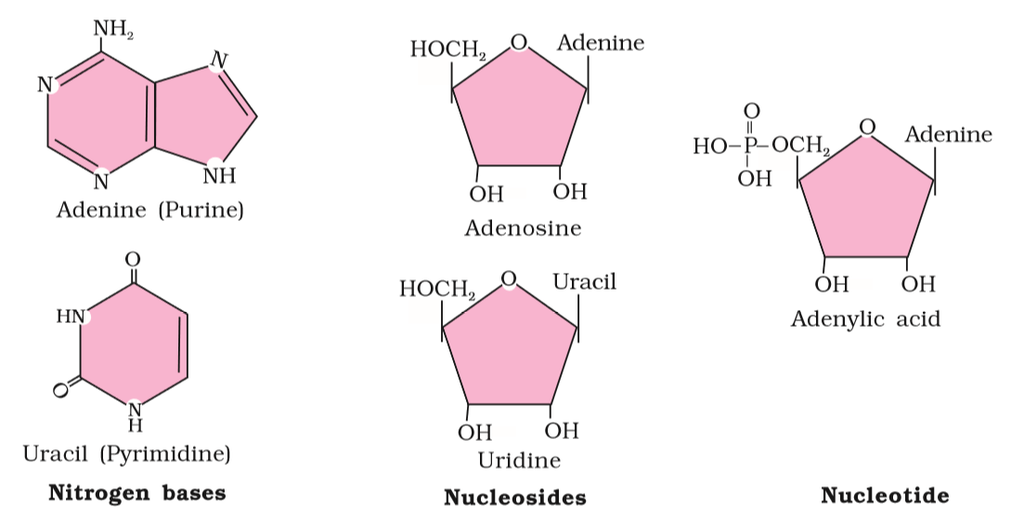
Figure 2(b): Diagrammatic representation of small molecular weight organic compounds in living tissues
Heterocyclic rings can be found in a variety of carbon compounds found in living beings. Adenine, guanine, cytosine, uracil, and thymine are examples of nitrogen bases. They're called nucleosides when they're found linked to sugar. Nucleotides are formed when a phosphate group is additionally esterified to the sugar. Nucleosides include adenosine, guanosine, thymidine, uridine, and cytidine. Nucleotides include adenylic acid, thymidylic acid, guanylic acid, uridylic acid, and cytidylic acid. Only nucleotides make up nucleic acids like DNA and RNA. DNA and RNA are two types of genetic material.
Primary and Secondary Metabolites
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PRIMARY AND SECONDARY METABOLITES
The most exciting aspect of chemistry deals with isolating thousands of compounds, small and big, from living organisms, determining their structure and if possible synthesising them.
If one were to make a list of biomolecules, such a list would have thousands of organic compounds including amino acids, sugars, etc.
We can call these biomolecules as 'metabolites'.
In animal tissues, one notices the presence of all such categories of compounds. For example, proteins, carbohydrates, fats, amino acids, nucleic acids.
These are called primary metabolites.
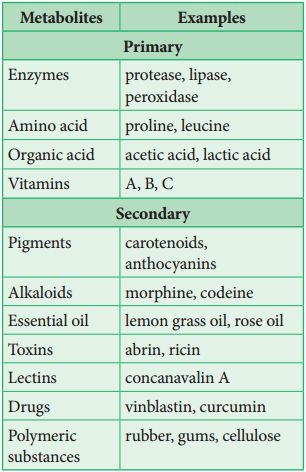
However, when one analyses plant, fungal and microbial cells, one would see thousands of compounds other than these primary metabolites which are called secondary metabolites, such as alkaloids, flavonoids, rubber, essential oils, antibiotics, coloured pigments, scents, gums and spices.
Difference between in Primary and Secondary Metabolites
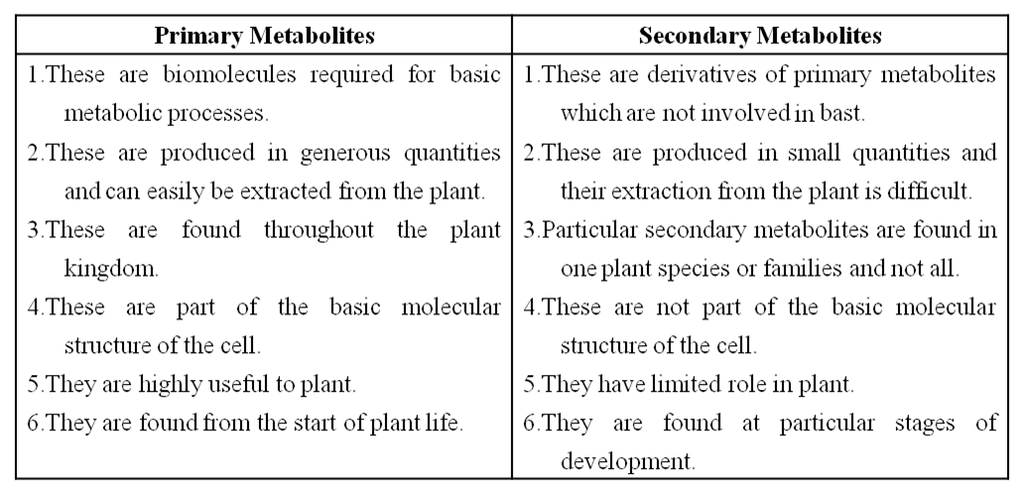
Primary metabolites have identifiable functions and play known roles in normal physiological processes.
While many of the secondary metabolites are useful to 'human welfare' (e.g., rubber, drugs, spices, scents and pigments) their physiological role is unknown.
Some secondary metabolites have ecological importance too.
Some Secondary Metabolites
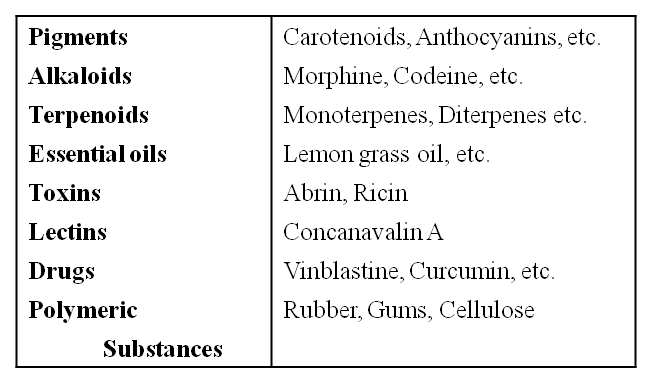
Let us take a detailed look at various micromolecules and macromolecules in a cell.
Primary and Secondary Metabolites
Primary and Secondary Metabolites
The most interesting component of chemistry is extracting thousands of small and large chemicals from live creatures, establishing their structure, and, if possible, synthesizing them. A list of biomolecules might contain thousands of organic chemicals, such as amino acids, carbohydrates, and other substances. These biomolecules can be referred to as metabolites. All of these kinds of chemicals can be found in animal tissues. Primary metabolites are what these are called. Thousands of additional substances termed secondary metabolites can be found in the plant, fungal, and microbial cells, such as alkaloids, flavonoids, rubber, essential oils, antibiotics, colored pigments, fragrances, gums, and spices. Secondary metabolites are what these are called.While primary metabolites have well-defined functions and roles in normal physiological processes, the role and functions of all secondary metabolites' in host organisms are currently unknown. Many of them, however, are beneficial to 'human welfare (e.g., rubber, drugs, spices, scents and pigments). Secondary metabolites play an important role in ecology.
Biomacromolecules
Proteins, nucleic acids, polysaccharides, and lipids are the only organic molecules found in the acid-insoluble fraction. With the exception of lipids, these substances have molecular weights in the tens of thousands of Daltons or higher. Biomolecules, or chemical substances found in living beings, are divided into two categories for this reason. One, those which have molecular weights less than one thousand daltons and are commonly referred to as micromolecules or simply biomolecules whereas those which are discovered in the acid-insoluble fraction are dubbed macromolecules or biomacromolecules. All of the chemicals in the acid-soluble pool have one thing in common. They have molecular weights that range from 18 to 800 daltons (Da).
With the exception of lipids, the molecules in the insoluble fraction are polymeric. Lipids are small molecular weight substances that can be found in a variety of forms, including cell membranes and other membranes. When the cell structure is disturbed and the tissue ispulverized, the breakdown of cell membranes and other membranes results in the formation of vesicles that are not water-soluble. As a result, membrane fragments in the form of vesicles separate from the acid-insoluble pool, resulting in the macromolecular fraction. The cytoplasmic composition is generally represented by the acid-soluble pool. The acid-insoluble fraction contains macromolecules from the cytoplasm and organelles. They represent the total chemical composition of biological tissues when put together.In summation, it is observed that water is the most abundant component in living organisms upon characterizing the chemical makeup of living tissue from the standpoint of abundance and classifying them accordingly.
Carbohydrates
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Carbohydrates
Carbohydrates are mainly compounds of carbon, hydrogen and oxygen.
Carbohydrates are so called because in most of them, the proportion of hydrogen and oxygen is the same as in water (H2O) i.e., 2 : 1.
These are also known as saccharides (compounds containing sugar).
Carbohydrates are produced by green plants during photosynthesis.
These constitute about 80% of the dry weight of plants.
Carbohydrates are divided into 3 main classes -monosaccharides, oligosaccharides and polysaccharides.
1. Monosaccharides
(i) These are single saccharide units with CnH2nOn general formula which cannot be hydrolysed further into still smaller carbohydrates. These are composed of 3-7 carbon atoms and are classified according to the number of C atoms as trioses (3C), tetroses (4C), pentoses (5C), hexoses (6C) and heptoses (7C). Of these, pentoses and hexoses are most common. Monosaccharides are important as energy sources and as building blocks for the synthesis of large molecules.
(ii) All monosaccharides are either aldoses or ketoses. Simplest monosaccharides include trioses e.g., glyceraldehyde and dihydroxyacetone.
(iii) Tetroses (e.g., erythrose) are rare. Erythrose takes part in the synthesis of lignin and anthocyanin pigments.
(iv) Ribose, ribulose, xylulose and arabinoses are pentoses. Xyluloses and arabinoses polymerise to form xylans and arabans which are cell wall material.
(v) Glucose, fructose, mannose, galactose are hexoses. These are white, sweet-tasting, crystalline and extremely soluble in water.
(vi) Glucose is called universal sugar and is also known as dextrose or grape sugar or corn sugar.
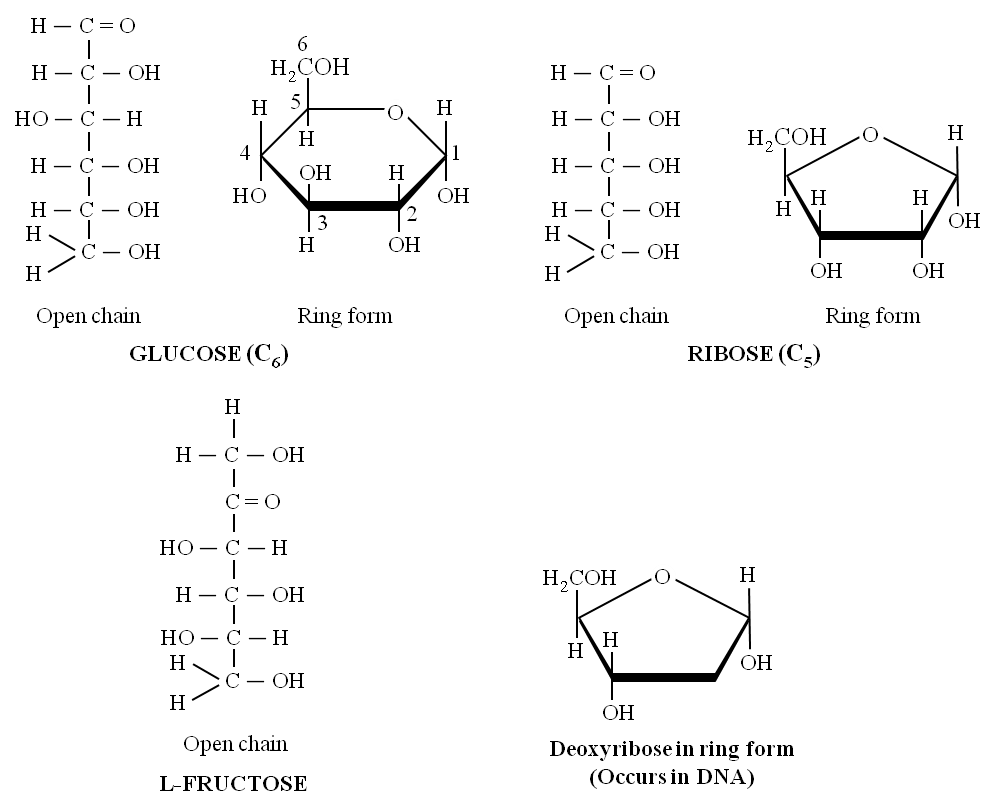
(vii) Fructose is called fruit sugar and is also known as levulose. It is the naturally occurring sweetest sugar. Honey has two sugars -Dextrose and Levulose.
(viii) Heptoses have 7 carbon atoms per molecule of sugar with general formula C7H14O7 e.g., sedoheptulose. It is an intermediate of respiratory and photosynthetic pathways.
Pentoses and hexoses of monosaccharides occur in solid forms i.e., open chain and ring chain. There are two types of ring chains i.e.,
(a) pyranose ring, which has hexagonal shape with 5 carbon atoms and one oxygen atom and
(b) furanose ring, which has pentagonal shape with 4 carbon atoms and one oxygen atom.
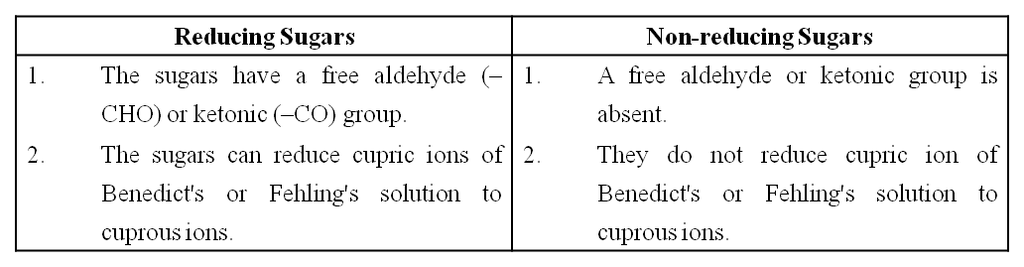
(ix) Monosaccharides have 'free' aldehyde or ketone group which can reduce Cu++ to Cu+. Hence, these are also called reducing sugars.
(x) Monosaccharides have two important chemical properties.
(a) Sugars having a free aldehyde or ketone group can reduce Cu++ to Cu+. These are called reducing sugars. This property is the basis of Benedict's test and Fehling's test to detect the presence of glucose in urine.
(b) The aldehyde or ketone group of monosaccharide can react and bind with an alcoholic group of another organic compound to join the two compounds together. This bond is called the glycosidic bond. This bond can be hydrolysed to give the original reactants.
Differences between Reducing and Non-reducing Sugar
Concept Builder
Derived Monosaccharides
(i) Deoxysugar -Loss of oxygen atom at 2nd carbon of ribose, yields deoxyribose, a constituent of DNA.
(ii) Amino sugar -Monosaccharides having an amino group e.g. glucosamine, galactosamine
(iii) Sugar acid -e.g., Ascorbic acid, glucuronic acid, galacturonic acid.
(iv) Sugar alcohol -e.g., glycerol and mannitol (present in brown algae).
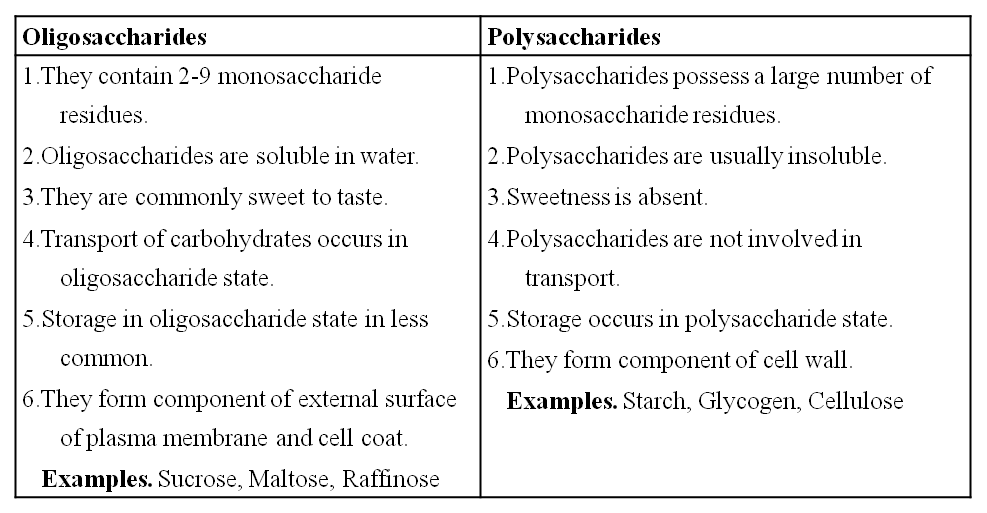
3. Oligosaccharides : They are condensation product of (2-9) monosaccharides. These include diasaccharides, trisaccharides, tetrasaccharides, hexasaccharides, heptasaccharides etc.
Differences between Oligosaccharides and Polysaccharides
(a) Disaccharides:
These are formed by condensation reactions between two monosaccharides (usually hexoses).
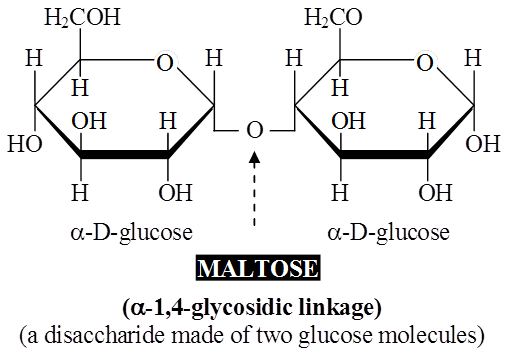
The bond formed between two monosaccharides is called a glycosidic bond.
It normally forms between C-atoms 1 and 4 of neighbouring units (1, 4 bond).
Once linked, the monosaccharide units are called residues.
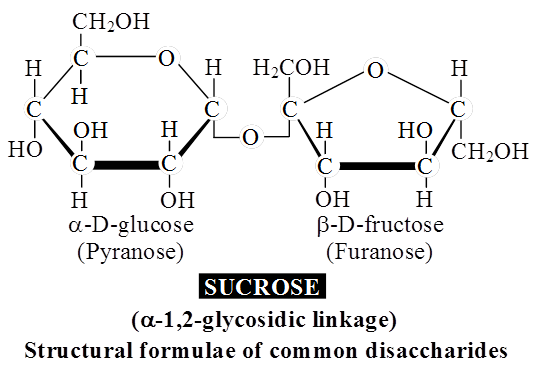
A molecule of sucrose is formed from a molecule of glucose and one of fructose.
Sucrose is the storage product of photosynthesis in sugarcane and sugarbeet.
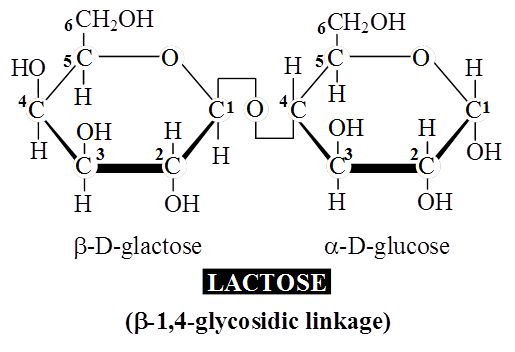
Lactose or milk sugar is found in human milk and cow's milk.
It is formed from one glucose molecule and one of galactose.
Maltose or malt sugar is formed from two molecules of glucose during germination of starchy seeds.
Maltose and lactose are reducing disaccharides.
Sucrose does not reduce Cu++ to Cu+, hence sucrose is a non-reducing sugar.
(b) Trisaccharides:
Sugars composed of 3 monosaccharide units are called trisaccharides (e.g. raffinose).
Raffinose is a common trisaccharide found in plants.
Upon hydrolysis, it yields one molecule each of glucose, fructose and galactose.
Larger oligosaccharides are attached to the cell membrane and enable the cell-cell recognition due to their presence.
They also take part in antigen specificity.
4. Polysaccharides
These are polymers of monosaccharides and are branched or unbranched linear molecular chains.
These are insoluble carbohydrates and are considered to be non-sugars.
Starch, glycogen, cellulose, pectin, hemicellulose, inulin are polysaccharides.
Body cells store carbohydrates as polysaccharides since these are easy to store and can be easily converted back into simple carbohydrates upon hydrolysis. These are in more condensed form and they have high molecular weight. These cannot pass through the plasma membrane.
Polysaccharides are of two types :
(i) Homopolysaccharides - consist of only one type of monosaccharide monomer e.g. starch, glycogen and cellulose, fructan, xylan, araban, galactan.
(ii) Heteropolysaccharides - consist of more than one type of monosaccharide monomer e.g. chitin, agar, arabanogalactans, arabanoxylans etc.
Polysaccharides are of three main types -storage (e.g. starch and glycogen), structural (e.g. chitin, cellulose) and mucopolysaccharides (e.g. keratan sulphate, chondroitin sulphate, hyaluronic acid, agar, alginic acid, carrageenin and heparin).
(a) Storage Polysaccharides
Food-Storage Polysaccharides: Starch is found abundantly in rice, wheat and other cereal grains legumes, potato, tapioca and bananas.
It is formed during photosynthesis and serves as an energystoring material.
Glycogen found in liver and muscles stores energy in mammals.
Storing carbohydrates in the form of polysaccharides has two advantages.
During their formation, many molecules of water are removed from monosaccharides.
This helps in condensing the bulk to be stored.
Unlike small carbohydrates, polysaccharides are relatively easy to store.
When necessary, polysaccharides are broken down by enzymes for the release of energy.
Starch
Starch, glycogen and inulin are reserve food materials.
Starch is a polymer of a-D-glucose. It is the major reserve food in plants.
Starch has two components -amylose (an unbranched polymer) and amylopectin (a branched polymer).
Amylopectin: Consists of 2000 -200,000 glucose molecules forming straight chain and shows branching (after 25 glucose units). Branching point has , 1-6 glycosidic linkage.
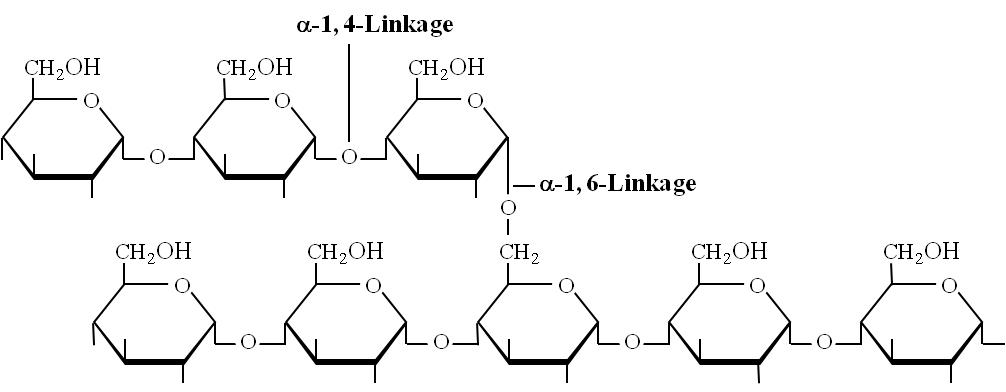
Amylopectin (branched polysaccharide)
Amylose: Consists of , 1-4 glycosidic linkage between -D glucose molecules. It is a straight chain of 200 -1000 glucose units. Starch forms helical secondary structures, each turn consists of 6 glucose units.
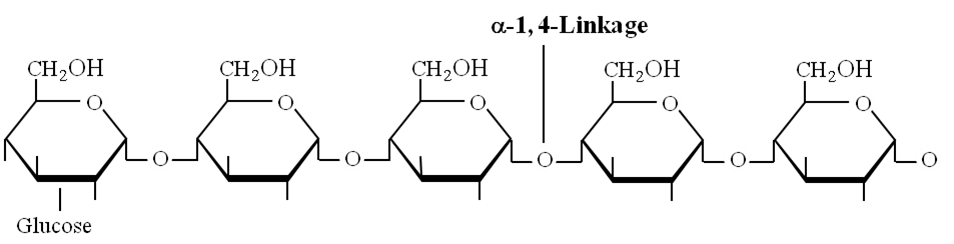
Structure of amylose showing -1, 4 linkage
Concept Builder
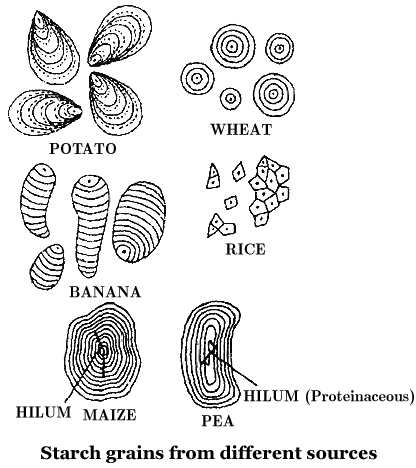
Starch molecules accumulate in the form of layers (stratifications) around a shifting organic centre (hilum) to form starch grains.
Hilum is made up of protein. In eccentric starch grains, hilum lies on one side.
These are found in potatoes.
In concentric starch grains, hilum is present in the centre.
These are found in wheat, maize, pea.
Dumb-bell shaped starch grains are found in the latex of Euphorbia.
Starch grains with single hilum are called simple (e.g. maize) but those with more than one hilum are called compound (e.g. potato, rice).
Starch turns blue with iodine as the helices in starch hold I2.
(ii) Glycogen: Glycogen is the animal equivalent of starch, many fungi also store it. Glycogen turns red-violet with iodine.
It consists of 30,000 glucose units joined by , 1-4 bonds, much more branched than starch. Branch point has , 1-6 linkages and branching occurs after 10-14 glucose units.
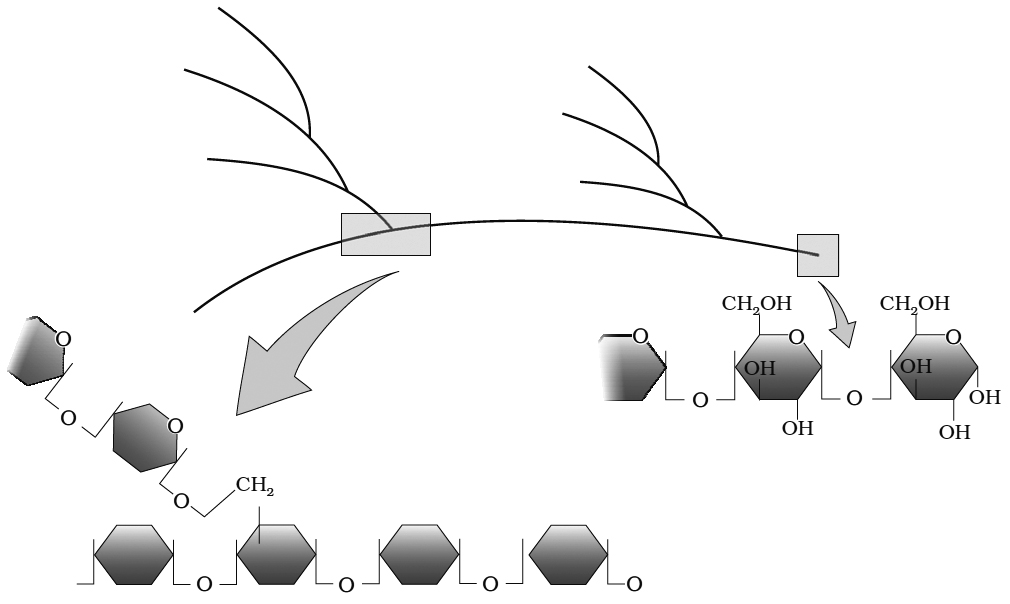
Diagrammatic representation of a portion of glycogen
(iii) Inulin: It is an unusual polysaccharide and polymer of fructose. It is stored particularly in roots and tubers of the family Compositae e.g. Dahlia tubers.
(b) Structural Polysaccharides Cellulose (Hexosan polysaccharide) :
Cellulose is the main structural unbranched homopolysaccharic of plants.
One molecule of cellulose has about 6000 -glucose residues.
Cotton fibres contain the largest amount (90 percent) of cellulose among natural materials.
Wood contains between 25 to 50 percent cellulose, the rest being hemicellulose and lignin.
Fibres of cotton, linen and jute are used for textile and ropes.
The artificial fibre Rayon is manufactured by dissolving cellulosic materials in alkali and by extruding and coagulating the filaments.
By treatment with other chemicals, cellulose is converted into Cellulose Acetate (used in fabrics, cellulosic plastics and shatter-proof glass), Cellulose Nitrate (used in propeliant explosives) and Carboxymethyl Cellulose (added to ice creams, cosmetics and medicines to emulsify and give a smooth texture).
Cellulose can be hydrolysed to soluble sugars.
Microbes can then convert these sugars to form ethanol, butanol, acetone, methane and other useful chemicals.
Cellulose is unbranched homopolysaccharide of -glucose.
Cellulose is the most abundant carbohydrate in biosphere.
Cellulose is produced by plants and is used for building cell walls. Cellulose is the most abundant organic compound in the biosphere.
Wood and cotton contain large quantities of cellulose.
Chitin is a polysaccharide found in the exoskeleton of insects, crabs and prawns.
Chitin is similar to cellulose in many ways except that its basic unit is not glucose, but a similar molecule that contains nitrogen (N-acetylglucosamine).
Although chitin is soft and leathery, it becomes hard when impregnated with calcium carbonate or certain proteins.
The insolubility of these polysaccharides in water helps to retain the form and strengthens the structure of organisms.
Pectin and hemicellulose: Pectin and hemicelluloses are structural polysaccharides.
Pectins are made up of arabinose, galactose and galacturonic acid.
Pectic acid is an acidic polysaccharide of methyl ester of D-galacturonic acid.
Middle lamella which binds the cells together is composed of calcium pectate.
Due to this substance, water absorption capacity of cell wall is increased.
Fruit walls contain high percentage of pectin.
During ripening, pectin breaks down into simple sugars resulting in the sweetening and loosening of fruits.
Hemicellulose is a mixture of D-xylose linked by 1-4 glycosidic bond.
Xylans, arabans, galactans are hemicelluloses. Food such as dates -Phoenix have hemicellulose as reserve food.
(c) Mucopolysaccharides
The slimy substances produced by plants are called mucilages.
When you soak the seeds of isabgol (Plantago ovata) or cut the fruit of okra (bhindi), you will notice the presence of a slimy substance.
Mucilages are polysaccharides formed from galactose and mannose.
Many seaweeds yield mucilages of commercial value such as agar, alginic acid and carrageenin.
Mucopolysaccharides are found in cell walls of bacteria and in the connective tissues of animals, as well as in body fluids.
These bind proteins in cell walls and connective tissue and water in interstitial spaces thereby providing lubrication in ligaments and tendons.
The vitreous humor of the eye and synovial fluid also contain mucopolysaccharides.
Hyaluronic acid is found in connective tissue and in cell walls.
Keratin sulphate and chondroitin sulphate occur in cartilage, cornea and the skin and impart strength and flexibility to them.
Keratan sulphate - consists of acetyl glucosamine, galactose and sulphuric acid, provides strength and flexibility to skin and cornea.
Hyaluronic acid -consists of D-glucuronic acid and N-acetyl glucosamine, present in the vitreous humor of eye, synovial fluid and cerebrospinal fluid etc.
Heparin is a polymer of sulfated glucosamine and sulfated iduronic acid.
It is an anticoagulant present in human blood.
Husk of Plantago ovata and mucilage of Aloe barbadensis are medicinally used.
Agar, alginic acid carrageenin are obtained from marine algae.
Artificial silk is polysaccharide prepared from rayon.
Carbohydrates
Carbohydrates
Carbohydrate is a group of organic compounds occurring in living tissues and foods in the form of starch, cellulose, and sugars. It is one of the three micronutrients via which a human body obtains energy. The properties of carbohydrate biology include carbon, hydrogen, and oxygen atoms at their chemical level. Cn(H2O)n is the generic formula for all carbohydrates. This formula is only valid for simple sugars, which are made up of the same amount of carbon and water.
There are two types of carbohydrates, simple and complex. This division is primarily based on their chemical structure along with their degree of polymerization.
Simple Carbohydrates: Simple carbohydrates carry one or two molecules of sugar. Such examples of carbohydrates are found abundantly in dairy products, refined sugar, etc. Since these carbohydrates do not comprise any fiber, vitamin, or mineral, they are regarded as empty calories. Simple carbohydrates can be further divided into three categories. These are as follows:
Monosaccharides: Carbohydrates consisting of one sugar molecule are called monosaccharides. Monosaccharides can be further classified based on the number of carbon atoms. These are trioses, tetroses, pentoses, hexoses, and heptoses. One of the most significant monosaccharides is glucose. The following are the two most frequent methods for preparing glucose. Sucrose is converted to glucose and fructose when it is cooked with dilute acid in an alcohol solution. From Starch, Glucose can also be made by hydrolyzing starch and boiling it with weak sulphuric acid at 393 degrees Fahrenheit under high pressure. Glucose, commonly known as dextrose and aldohexose, is abundant on the planet.
Disaccharides: Two monosaccharides combine to form a disaccharide. Sucrose, Lactose, and Maltose are some of the prime examples of this carbohydrate.
Oligosaccharides: Carbohydrates consisting of 2-9 monomers are classified as oligosaccharides.
The term “monosaccharide” refers to a carbohydrate derivative possessing a single carbon chain; “disaccharide” and “trisaccharide” refer to molecules containing two or three such monosaccharide units joined together by acetal or ketal linkages. “Oligosaccharide” and “polysaccharide” refer to larger such aggregates, with “a few” and many monosaccharide units, respectively. Current usage seems to draw the distinction between “few” and many at around 10 units.
Complex Carbohydrate: Complex carbohydrates are made up of two or more molecules of sugar. Such carbohydrates are found abundantly in food items like corn, lentils, peanuts, beans, etc. Complex carbohydrates are also known as polysaccharides as they are formed due to polymerization.
Polysaccharides are another macromolecule family found in the acid-insoluble pellet. Polysaccharides are lengthy sugar chains. They're threads (literally, cotton threads) made up of various monosaccharides as building blocks. cellulose, for example, is a polymeric polysaccharide made up of only one type of monosaccharide, glucose. Cellulose is a homopolymer, which means it is made up of only one type of molecule. Starch is a type of this that is found in plant tissues as a source of energy. Glycogen is a different type of carbohydrate found in animals. Inulin is a fructose polymer. The right end of a polysaccharide chain (such as glycogen) is known as the reducing end, while the left end is known as the non-reducing end. It has branches. Secondary helical structures are formed by starch.In fact, the helical part of starch can retain I2 molecules. The colour of starch-I2 is blue. Because cellulose lacks complex helices, it is unable to retain I2.
Cellulose is the main component of plant cell walls. Cellulosic paper is created from plant pulp and cotton fibre. In nature, there exist more complicated polysaccharides. Amino-sugars and chemically modified sugars are used as building blocks (e.g., glucosamine, N-acetyl galactosamine, etc.). Arthropod exoskeletons, for example, contain a complex polymer called chitin. The majority of these complex polysaccharides are homopolymers.
Amino Acids
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
AMINO ACIDS
Amino acids are small molecules made of carbon, hydrogen, oxygen and nitrogen and in some cases also sulphur.
Each amino acid has a free amino group, a free carboxyl group and 'R' as side chain as same substituents on same carbon atom.
Amino group leads basic character while carboxylic group leads acidic character to the molecule.
Lysine and arginine are Basic Amino Acids because they carry two amino groups and one carboxylic group.
Glutamic acid (glutamate) and aspartic acid (aspartate) contain one amino and two carboxyl groups each and are classified as Acidic Amino Acids.
Alanine, glycine, valine are Neutral Amino Acids as these contain one amino and one carboxyl group each.
These are 20 different amino acids coded by our DNA that differ in the side chain.
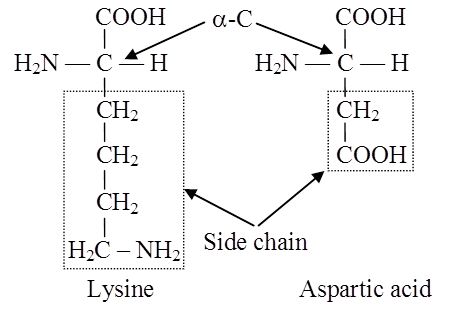
Side chain of a basic and an acidic amino acid
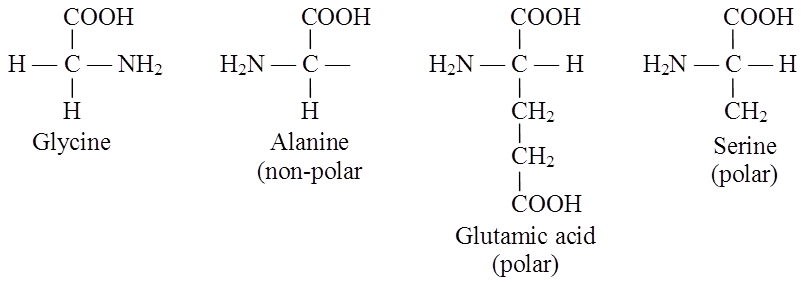
Examples of polar and nonpolar amino acids
Most amino acids are laevo-rotatory while glycine is optically inactive.
There are three important non-protein amino acids.
They are ornithine, citrulline (both are involved in ornithine cycle to synthesise urea) and diaminopimelic acid.
A particular property of amino acids is the ionizable nature of -NH2 and -COOH groups.
Hence, in solutions of different pH, the structure of amino acids changes.
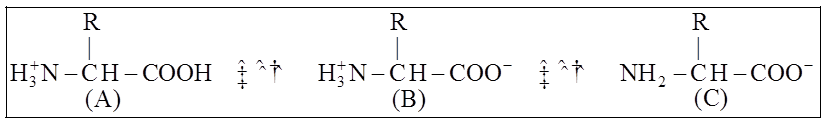
B is called Zwitter ionic form
There are two types of amino acids viz. essential and non-essential amino acids.
Essential amino acids cannot be synthesized by animals whereas non-essential amino acids can be synthesised in animal's body.
There are seven essential amino acids in animals whereas 8 essential amino acids in man.
These are leucine, isoleucine, valine, tryptophan, phenylalanine, lysine and methionine.
Threonine is an additional essential amino acid in human beings.
Two amino acids viz. arginine and histidine are semi-indispensable amino acids as they can be synthesised by human beings but very slowly.
Differences between Essential and Nonessential Amino Acids

Concept Builder
Amino acids are classified into following groups :
(i) Neutral amino acid: With one -NH2 and one -COOH group e.g. glycine, alanine (non-polar).
(ii) Acidic amino acid: Have an extra COOH group (monoamino dicarboxylic), e.g. glutamic and aspartic acid.
(iii) Basic amino acid: Have additional NH2 group (diamino monocarboxylic) e.g. arginine, lysine.
(iv) Sulphur containing amino acid: Have sulphur e.g. cysteine, cystine and methionine.
(v) Alcoholic amino acid: Have -OH group e.g. serine, threonine.
(vi) Aromatic amino acid : Have cyclic structure having a side chain with -COOH and NH2 groups e.g. phenyl alanine, tryptophan, tyrosine.
(vii) Heterocyclic amino acid: N is present in the ring e.g. proline, histidine, hydroxyproline.
(viii) Semi essential amino acid : Arginine and histidine are semi-essential amino acids required by children.
Protein amino acids are laevorotatory and -type except glycine. (Glycine: Simplest amino acid, involved in the formation of heme).
Functions of Amino Acids
Besides their principal function as building blocks for proteins, specific amino acids are also converted into different types of biologically active compounds.
For example, tyrosine is converted into the hormones thyroxine and adrenaline, as well as the skin pigment melanin, glycine is involved in the formation of heme and tryptophan in the formation of the vitamin nicotinamide as well as the plant hormone indole-3-acetic acid.
After the removal of the amino group the carbon chain of many amino acids is converted into glucose.
On losing the carboxyl groups as carbon dioxide, amino acids form biologically active amines such as histamine. Histamine is required for the functioning of muscles, blood capillaries and gastric juices.
Ornithine and citrulline are components of urea cycle.
Antibiotics contain non protein amino acids.
Amino acids form organic acids which form glucose by gluconeogenesis.
Lysine is an essential amino acid because it is not formed in the body and has to be provided through diet.
Amino Acids
Amino Acids
Amino acids are chemical molecules with amino and carboxylate functional groups as well as a side chain that is unique to each amino acid. Amino Acids are chemical substances that combine to produce proteins, which is why they are known as the building blocks of proteins. These biomolecules have a role in a variety of biological and chemical functions in the human body and are essential for human growth and development. There are around 300 amino acids found in nature. Amino acids' general features include:
1. A high melting and boiling point.
2. Amino acids are crystalline solids that are white in color.
3. Few amino acids have a pleasant, tasteless, or bitter flavour.
4. The majority of amino acids are water-soluble and insoluble in organic solvents.
There are 20 amino acids found in nature, all of which have the same structural features: an amino group (-NH3+), a carboxylate group (-COO-), and a hydrogen-bonded to the same carbon atom. Their side-chain, known as the R group, distinguishes them from one another. The - carbon of each amino acid is connected to four distinct groups namely: Amino group, COOH, Hydrogen atom, and Sidechain.
Amino acids have a general structure which can be represented as:
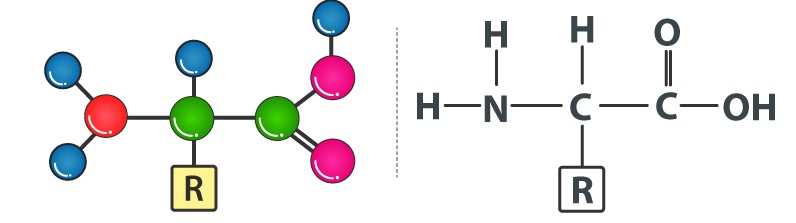
Our bodies can easily produce a few non-essential amino acids out of a total of 20 amino acids. Alanine, asparagine, arginine, aspartic acid, glutamic acid, cysteine, glutamine, proline, glycine, serine, and tyrosine are examples of these amino acids.
Besides these, there are nine other amino acids that are extremely important because our bodies cannot make them. Isoleucine, histidine, lysine, leucine, phenylalanine, tryptophan, methionine, threonine, and valine are examples of important amino acids.
Amino acids are essential for a variety of biological and chemical tasks in our bodies, including tissue construction and repair, enzyme production and activity, food digestion, molecule transportation, and so on. Only a few amino acids can be synthesized by our bodies, thus the rest, known as essential amino acids, must be obtained from protein-rich foods in our daily diet. Plant-based foods with high levels of amino acids include broccoli, beans, beets, pumpkin, cabbage, almonds, dry fruits, chia seeds, oats, peas, carrots, cucumber, green leafy vegetables, onions, soybeans, whole grain, peanuts, legumes, lentils, and so on. Apples, bananas, berries, figs, grapes, melons, oranges, papaya, pineapple, and pomegranates are high in amino acids.Dairy products, eggs, seafood, poultry, beef, pork, and other animal products are examples of other animal goods.
Functions of Essential Amino acids
1. Phenylalanine helps in maintaining a healthy nervous system and in boosting memory power.
2. Valine acts as an important component in promoting muscle growth.
3. Threonine helps in promoting the functions of the immune system.
4. Tryptophan is involved in the production of vitamin B3 and serotonin hormones. This serotonin hormone plays a vital role in maintaining our appetite, regulating sleep and boosting our moods.
5. Isoleucine plays a vital role in the formation of hemoglobin, stimulating the pancreas to synthesize insulin, and transporting oxygen from the lungs to the various parts.
6. Methionine is used in the treatment of kidney stones, maintaining healthy skin and also used in controlling invade of pathogenic bacteria.
7. Leucine is involved in promoting protein synthesis and growth hormones.
8. Lysine is necessary for promoting the formation of antibodies, hormones, and enzymes and in the development and fixation of calcium in bones.
9. Histidine is involved in many enzymatic processes and in the synthesizing of both red blood cells (erythrocytes) and white blood cells (leukocytes).
Functions of Non-Essential Amino acids
1. Alanine functions by removing toxins from our body and in the production of glucose and other amino acids.
2. Cysteine acts as an antioxidant and provides resistance to our body; it is important for making collagen. It affects the texture and elasticity of the skin
3. Glutamine promotes a healthy brain function and is necessary for the synthesis of nucleic acids – DNA and RNA.
4. Glycine is helpful in maintaining the proper cell growth, and its function, and it also plays a vital role in healing wounds. It acts as a neurotransmitter.
5. Glutamic acid acts as a neurotransmitter and is mainly involved in the development and functioning of the human brain.
6. Arginine helps in promoting the synthesis of proteins and hormones, detoxification in the kidneys, healing wounds, and maintaining a healthy immune system.
7. Tyrosine plays a vital role in the production of the thyroid hormones -T3 and T4, in synthesizing a class of neurotransmitters and melanin, which are natural pigments found in our eyes, hair, and skin.
8. Serine helps in promoting muscle growth and in the synthesis of immune system proteins.
9. Asparagine is mainly involved in the transportation of nitrogen into our body cells, formations of purines and pyrimidine for the synthesis of DNA, the development of the nervous system and improving our body stamina.
10. Aspartic acid plays a major role in metabolism and in promoting the synthesis of other amino acids.
11. Proline is mainly involved in the repairing of the tissues and the formation of collagen, preventing the thickening and hardening of the walls of the arteries (arteriosclerosis) and in the regeneration of new skin.
Proteins
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
PROTEINS
Berzelius coined the term protein. Proteins are hetero polymers of amino acids.
Two amino acids can join through amino group of one and carboxylic group of the other forming an anhydro bond (CO-NH linkage) also known as peptide bond by loss of water molecule.
A protein is a heteropolymer and not a homopolymer.
Collagen is the most abundant protein in animal world and Rubisco (Ribulose biphosphate carboxylase oxygenase) is the most abundant protein in the whole biosphere.
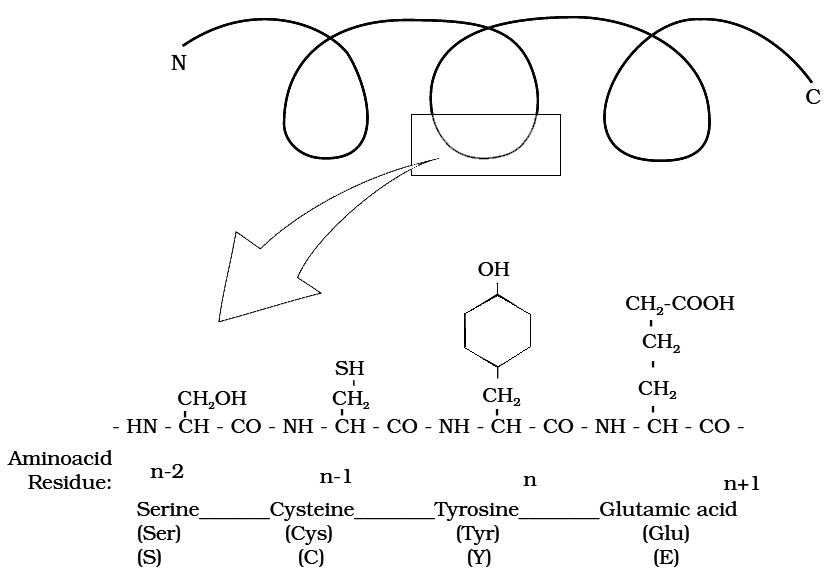
Primary structure of a portion of a hypothetical protein.
N and C refer to the two termini of every protein. Single letter codes and
three letter abbreviations of amino acids are also indicated.
Structure of Proteins :
The four levels of protein structure are:
1. Primary Structrure :
The sequence of amino acids in polypeptide chain gives the protein its Primary Structure.
The primary structure is very important as it determines the specificity of protein but does not make a protein functional.
To be functional the protein must have a particular 3-dimensional structure (conformation).
A functional protein contains one or more polypeptide chains.
The sequence of amino acids in the chain determines where the chain will bend or fold and where the various lengths will be attracted to each other.
2. Secondary Structure :
Through the formation of hydrogen bonds, peptide chains assumes a Secondary Structure.
When a chain is arranged like a coil it is called an Helix.
When two or more chains are joined together by intermolecular hydrogen bonds, the structure is called Pleated Sheet.
Helical structure is found in keratin of hair and pleated structure found in silk fibres.
Each protein has a specific secondary structure also.
(a) It generailly takes the form of an extended spiral spring, the -helix, whose structure is maintained by many hydrogen bonds which are formed between adjacent -CO and -NH groups. The H atom of the NH group of one amino acid is bonded to the O atom of the CO group three amino acids away. A protein which is entirely helical is keratin.
(b) The other type of secondary structure is called -pleated sheet. Here, two or more chains are joined together by intermolecular hydrogen bonds as in silk fibres.
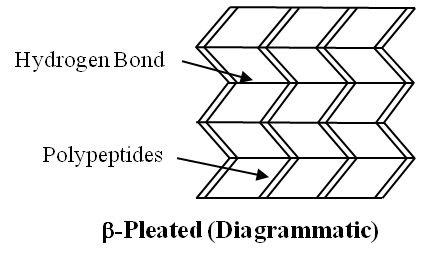
(c) A special secondary structure is observed in collagen or tropocollagen helix which has three strands or polypeptides coiled around one another. The coil is strengthened by the establishment of hydrogen bond between -NH group of glycine residue of each strand with -CO group of the other two strands. Locking effect is due to the proline and hydroxyproline.
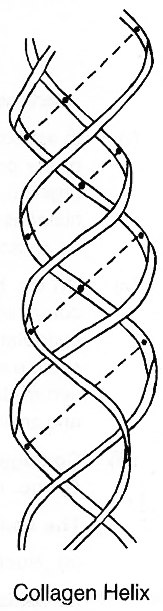
3. Tertiary structure :
Usually, the polypeptide chain bends and folds extensively and forms a compact 'globular' shape to obtain functional conformation. This is termed as the tertiary structure.
 Various types of bonds or interactions found during coiling of polypeptide
Various types of bonds or interactions found during coiling of polypeptide
In a large protein like haemoglobin, or in case of an enzyme, the molecule undergoes further folding and coiling to attain functional conformation.
The coils and folds of the protein molecule are so arranged as to hide non-polar amino acid side chains inside and expose the polar side chains.
The 3-dimensional conformation of a protein brings distant amino acid side chains closer.
The active sites of proteins such as enzymes are thus formed.
The conformation of proteins is easily changed by pH, temperature and chemical substances and hence the function of proteins is liable and subject to regulation.
4. Quarternary strucrure :
Many highly complex proteins consist of an aggregation of polypeptide chains held together by hydrophobic interactions and hydrogen and ionic bonds.
Their precise arrangement constitutes the quaternary structure.
Cartoon showing: (a) A secondary structure and (b) A tertiary structure of proteins
In aqueous media, proteins carry both cationic and anionic groups on the same molecule.
The ionic state of the protein depends on the pH of the medium.
A protein, rich in basic amino acids like lysine and arginine, exists as a cation and behaves as a base at the physiological pH of 7.4 (Basic Protein) e.g., histones of nucleoproteins.
Similady, a protein with acidic amino acids exists as an anion and behaves as an acid e.g., most blood proteins (Acidic Proteins).
Types of Proteins :
On the basis of constitution, proteins are classified as simple or conjugated.
1. Simple Protein :
Simple Proteins are composed of amino acids only.
Some are small, globular molecules mostly soluble in water and not coagulated by heat (e.g., histones).
As the size of the protein molecule increases, it becomes less soluble and its heat-coagulability increases.
For example, larger globular proteins (like egg albumin, serum globulins and glutelins of wheat or rice) are coagulated by heat.
Fibrous proteins have long molecures and are insoluble in water (e.g., keratin of skin and hair and collagen of connective tissues).
2. Conjugated proteins :
Conjugated Proteins are formed by binding of a simple protein with a non-protein called tile Prosthetic Group. e.g., Nucleoproteins have nucleic acids as prosthetic group.
The conjugated proteins are of following types :
(a) Nucleoproteins (prosthetic group-nucleic acid) e.g. protamines
(b) Metalloproteins (prosthetic group-metals) e.g. haemoglobin
(c) Chromoproteins (prosthetic group-pigment) e.g. cytochromes
(d) Phosphoproteins (prosthetic group-phosphoric acid) e.g. Casein of milk.
(e) Lipoproteins (prosthetic group-lipids) e.g. chylomicrons, HDL, LDL etc.
(f) Glycoproteins (prosthetic group-carbohydrates) e.g. mucins
Glycoproteins (and glycolipids) play an important role in cell recognition.
The specificity of this recognition depends upon the particular sequence of sugars in carbohydrate portions.
Ribulose biphosphate carboxylase (an enzyme) present in large amounts in chloroplast stroma is the world's most common protein.
Storage Proteins include albumin of egg and those that occur in seeds (glutelin of wheat). Prolamines are storage proteins.
Protamines are basic proteins associated with DNA of chromosomes, they are rich in lysine and arginine.
P-proteins are involved in the transport of organic compounds through phloem.
Keratin and fibroin form protective structures.
Antibodies are defence proteins.
Snake venom, ricin of castor and bacterial toxins are proteinaceous in nature.
Actin and myosin are essential for muscle contraction.
Microtubules have tubulin protein.
Haemoglobin and myoglobin are transport proteins.
Ovalbumin and glutelin are storage proteins present in cereals. Ferretin is iron storing protein of animal tissues. The type of prolamines and glutelins found in wheat are gliadin and glutenin.
Insulin and parathormone are proteinaceous hormones.
Fibrinogen and thrombin are blood clotting prbteins.
Rhodopsin and iodopsin are photoreceptor pigments. These are present in rods and cones of retina and are proteins.
Proteins having all essential amino acids are called first class proteins.
Monellin, a protein, is the sweetest chemical obtained from an African berry.
Cheese is a denatured protein.
Resilin -is a perfectly elastic protein found in wings of some insects.
Some proteins and their Functions
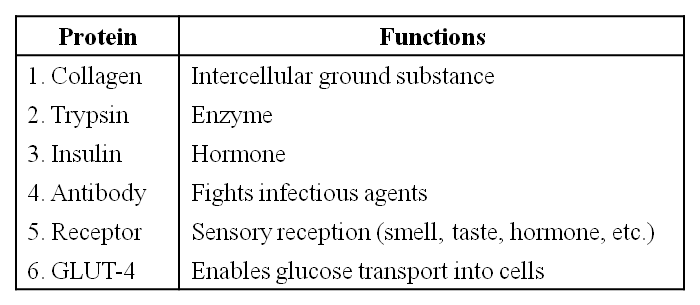
Proteins
Proteins
Polypeptides are the building blocks of proteins. They are peptide bonds that connect linear chains of amino acids. A polymer of amino acids makes up each protein. A protein is a heteropolymer, not a homopolymer because there are 20 different types of amino acids. A homopolymer is made up of only one type of monomer that repeats 'n' times. Specific amino acids are necessary for human health and must be obtained through our food. As a result, necessary amino acids are obtained from food proteins. As a result, amino acids can be classified as either essential or non-essential. The latter are those that our bodies can produce, whereas essential amino acids must be obtained through our diet.Proteins have a variety of activities in living creatures, including transporting nutrients across cell membranes, fighting pathogenic organisms, acting as hormones, and enzymatic reactions. The most abundant protein in the animal kingdom is collagen, and the most abundant protein in the biosphere is Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO).
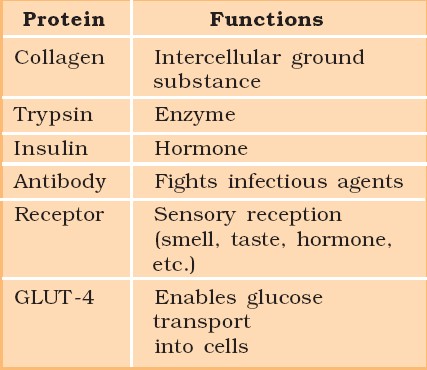
Structure of Proteins:
Proteins are heteropolymers made up of strings of amino acids, as previously stated. In different situations, the structure of molecules means different things. Physicists visualize molecular structures in three dimensions, while biologists explain protein structure on four levels. The primary structure of a protein is the sequence of amino acids, or the positional information in a protein — which amino acid is the first, which is the second, and so on.A protein can be visualized as a line, with the initial amino acid on the left end and the last amino acid on the right.
N-terminal amino acid is another name for the first amino acid. The C-terminal amino acid is the last one in the chain. As an extended stiff rod, a protein thread does not exist throughout. The thread is folded into a helix shape (similar to a revolving staircase). Naturally, only a piece of the protein thread is organized in a helix shape. Only right-handed helices are found in proteins. In what is known as the secondary structure, various portions of the protein thread are folded into other configurations.
Furthermore, the lengthy protein chain folds in on itself like a hollow woollen ball, forming the tertiary structure. This allows us to see a protein in three dimensions. Proteins' tertiary structure is required for many of their biological functions. Some proteins are made up of several polypeptides and subunits. The architecture of a protein, also known as the quaternary structure of a protein, is the way these individual folded polypeptides or subunits are arranged with regard to one other (e.g., a linear string of spheres, spheres placed one upon another in the form of a cube or plate, etc.).Adult human haemoglobin consists of 4 subunits. Two of these are identical to each other. Hence, two subunits of α type and two subunits of β type together constitute the human haemoglobin (Hb).
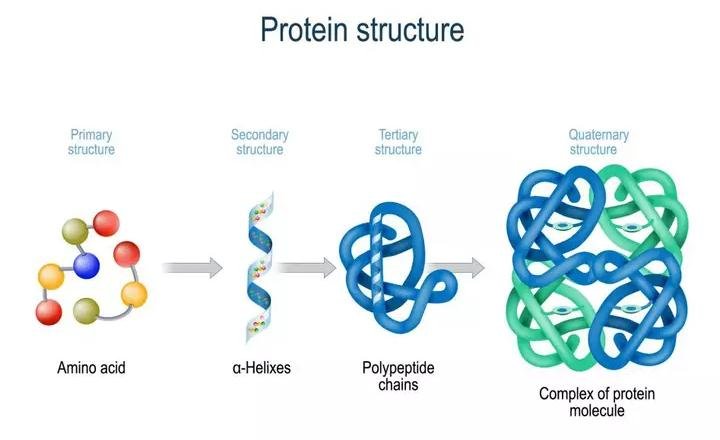
Nature of bond linking monomers in a polymer
A peptide bond is produced when the carboxyl (-COOH) group of one amino acid combines with the amino (-NH2) group of the next amino acid with the elimination of a water molecule in a polypeptide or protein (the process is called dehydration). Individual monosaccharides in a polysaccharide are joined by a glycosidic bond. Dehydration also contributes to the formation of this link. Two carbon atoms from two neighboring monosaccharides make a peptide bond. A phosphate moiety of a nucleic acid connects the 3'-carbon of one sugar of one nucleotide to the 5'-carbon of the sugar of the next nucleotide. An ester link exists between the phosphate and the sugar's hydroxyl group.The phosphodiester bond is named after the fact that there is one such ester bond on each side.
Lipids
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
LIPIDS
They are made of carbon, hydrogen and little oxygen.
The number of oxygen atoms in a lipid molecule is always less as compared to the number of carbon atoms.
Sometimes small amounts of phosphorus, nitrogen and sulphur are also present.
Lipids are insoluble in water, but soluble in non-polar solvents like chloroform and benzene. lipids contain fatty acids which may be saturated or unsaturated.
Fatty acids are organic acids with a hydrocarbon chain ending in a carboxyl group (COOH).
Fatty acids are called saturated if they do not have any double bonds between the carbons of the molecular chain e.g., palmitic acid (16 C) and stearic acid (18 C).
Their melting point is high.
CH3(CH2)14COOH CH3(CH2)16COOH General formula of saturated fatty acids
Palmitic acid Stearic acid CnH2nO2
Unsaturated Fatty acids have one or more double bonds between the carbons of the chain.
The 18 C unsaturated fatty acids oleic, linoleic and linolenic acids have 1, 2 and 3 double bonds respectively.
CH3(CH2)7CH = CH(CH2)7COOH General formula of unsaturated fatty acids
Oleic acid CnH2n-2xO2
(where x = number of double bonds)
Arachidonic fatty acid has 4 double bonds.
They have a bend at each double bond which keeps them in liquid form at ordinary temperature.
They are called polyunsaturated fatty acids (PUFA) when they have more than one double bond in them.
They are also called drying oils because they have a tendency to solidify on exposure.
Oils of groundnut, mustard seed, sesame seed and sunflower are rich in unsaturated fatty acids.
The unsaturated fatty acids have lower melting points than saturated fatty acids.
In lipids, fatty acids are usually in the form of esters.
Just as acids and bases react to form salts, similarly organic acids react with alcohol to form esters. Here alcohol is glycerol.
Plants can synthesize all fatty acids.
Animals can not synthesize linoleic, linolenic and arachidonic acid.
These are called essential fatty acids. Their deficiency causes sterility, kidney failure and stunted growth.
Differences between Saturated fatty acids & Unsaturated fatty acids
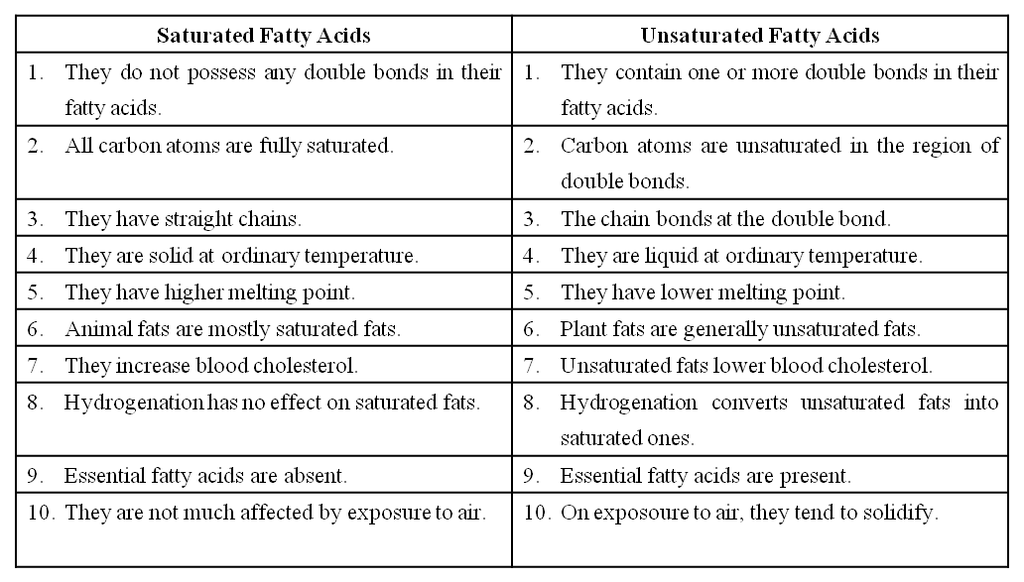
Lipids can be classified as :
1. Simple lipids:
Esters of fatty acids with alcohol.
Simplest alcohol in fats is glycerol (trihydroxypropane).
For example, fats, oils and waxes.
Triglycerides are common in nature.
Fats are esters of fatty acids with glycerol (glycerine).
Each molecule of glycerol can react with three molecules of fatty acids.
Depending on the number of fatty acids that are attached to the glycerol molecule, the esters are called mono-, di-or tri-glycerides.
Fats that are generally liquid at room temperature are called oils.
Oil's are rich in unsaturated fatty acids and consequently have low melting points.
On hydrogenation, the unsaturated fatty acids become saturated and the oil becomes a solid fat ("Vanaspati" and margarine).
(i) Waxes are another class of simple lipids. They are formed by combination of a long-chain fatty acid with a long chain alcohol. Waxes play an important role in protection. They form water-insoluble coatings on hair and skin of animals and stems, leaves and fruits of plants.
(ii) Bees wax is formed from palmitic acid (C16H32O2) and mericyl alcohol (C30H61OH). Bee wax is also called as Hexacosyl palmitate, secreted by worker bees. Lanolin (wool fat), forms a water proof coat around the animal fur.
(iii) Bacteria that cause tuberculosis and leprosy produce a wax (wax-D) that contributes to their pathogenicity.
Cutin is formed by cross esterification and polymerisation of hydroxy fatty acids and other fatty acids without esterification by alcohols other than glycerol. Cuticle has 50 -90% cutin.
Suberin is condensation product of glycerol and phellonic acid. It makes the cell wall impermeable to water.
2. Compound lipids:
These lipids contain an additional group alongwith fatty acids and alcohols e.g. phospholipids, glycolipids, lipoproteins and chromolipids.
(i) Phospholipids:
These are straight chain compounds of glycerol, fatty acids and phosphoric acid.
In these, only two fatty acids are attached to the glycerol molecule and the third hydroxyl group of glycerol is esterified to phosphoric acid instead of fatty acid.
Depending upon the type of phospholipid, this phosphate is also bound to a second alcohol molecule which can be choline, ethanolamine, inositol or serine.
Common phospholipids are lecithin and cephalin.
Phospholipids are amphipathic molecules having hydrophilic (water loving) polar region and hydrophobic (water repelling) non-polar regions.
They are the basic constituents of biomembranes.
Many phospholipids arrange themselves in a double layered membrane in aqueous media (lipid bilayer).
Cephalin is found in the brain and acts as insulation material for nerves and also participates in blood coagulation.
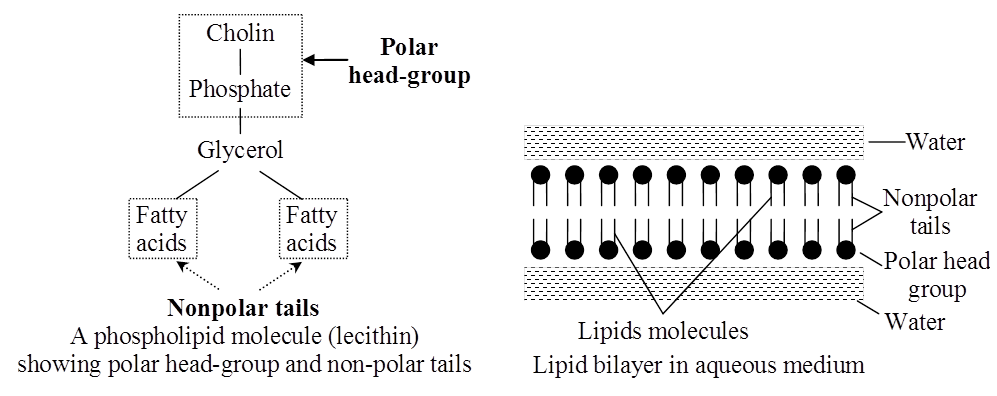
Lecithin takes part in cell permeability, osmotic tension and surface conditioning of cells.
The hydrocarbon chains of the fatty acids are the Non-Polar Tails of the molecule.
The phosphate and the nitrogenous/non-nitrogenous groups form the polar Head-Group of the molecule.
Many phospholipid molecules may arrange themselves in a double-layered membrane (Lipid Bilayer) in aqueous media. These have one or more simple sugars.
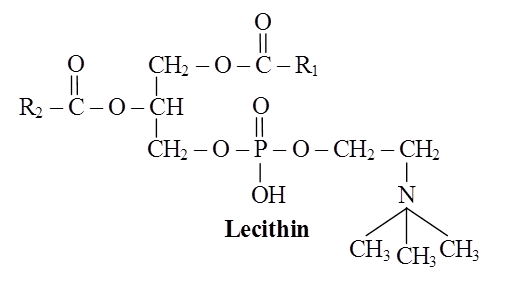
Structure of a phospholipid molecule (R1 and R2 are fatty acids)
(ii) Glycolipids:
They are lipids having sugar residues. Two common glycolipids are cerebrosides and gangliosides.
Composition : Glycolipids contain fatty acids, alcohol sphingosine and sugar such as galactose, glucose etc.
Function : The glycolipids are components of cell membranes, particularly in myelin sheath of nerve fibres and on outer surfaces of nerve cell and in chloroplast membranes.
(iii) Lipoproteins:
Lipoproteins contain lipids (mainly phospholipids) and proteins in their molecules.
Function: Membranes are composed of lipoproteins. Lipids are transported in the blood plasma and lymph as lipoproteins. Lipoproteins occur in the milk and egg yol'k.
(iv) Chromolipids:
These contain pigments such as carotenoids e.g. carotene, vitamin A.
3. Derived lipids –
These are isoprenoid structures e.g. steroids, terpenes, carotenoids, prostaglandins.
(i) Sterols:
Sterols belong to a class of lipids which are not straight chain compounds.
These are composed of fused hydrocarbon rings and a long hydrocarbon side chain.
One of the example is cholesterol.
The cholesterol is found in animals only.
It exists either free or as Cholesterol Ester with a fatty acid.
Cholesterol is also the precursor of hormones such as progesterone, testosterone, estradiol and cortisol.
Another steroid compound, diosgenin produced by the yam plant (Oioscorea) is used in the manufacture of antifertility pills.
(ii) Prostaglandins:
It is a group of hormone-like unsaturated fatty acids which function as messenger substances between cells.
They are derived from arachidonic acid and related C20 fatty acids.
Prostaglandins occur in human seminal fluid menstrual fluid, amniotic fluid and a number of tissues.
They also circulate in blood.
They produce a variety of effects in different organs.
(a) Prostaglandins regulate production of acid in stomach and stimulate contraction of smooth muscles.
(b) They are used to induce labour because they cause uterine contractions.
(c) These can reduce the effect of asthma and gastric acidity. Analgesics like aspirin inhibit prostaglandin synthesis.
(iii) Cholesterol helps in absorption of fatty acids, formation of sex hormones, vitamin D and bile salts. Potato is rich in cholesterol.
(iv) Terpenes are lipid like carbohydrates formed of isoprene units (C5H8)n, e.g., menthol, camphor, carotenoids.
NUCLEOTIDES
(i) This is a group of small complex molecules forming a part of the information transfer system in cells. They are basic units of nucleic acids. They also participate in energy transfer systems
(ii) Nucleotides contain carbon, hydrogen, oxygen, nitrogen and phosphorus.
Each nucleotide is made up of a cyclic nitrogenous base, a pentose and one to three phosphate groups.
The nitrogenous bases occurring in nucleotides are either a purine or a pyrimidine.
Major purines are adenine and guanine. Thymine, uracil and cytosine are pyrimidines.
The sugar pentose is either ribose or deoxyribose.
The nucleotides are thus called Ribonucleotides or Deoxyribonucleotides.
Examples of ribonucleotides and deoxyribonucleotides are adenylic acid (AMP) and deoxyadenylic acid (d AMP) repetitively.
Ribonucleotides are the basic units of ribonucleic acids (RNA) and deoxyribonucleotides are basic units of deoxyribonucleic acids (DNA).
Nucleotides are mono-, di-or tri-phosphate of nucleosides.
For example, adenylic acid or adenosins monophosphate (AMP).
Adenosine disphosphate (ADP) and adenosine triphosphate (ATP) are higher adenine nucleotides.
Nucleotides with more than one phosphate group are called higher nucleotides, e.g., ATP and ADP. likewise, other purines and pyrimidines can also form higher nucleotides.
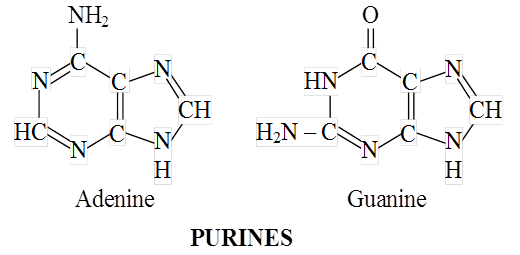
(iii) Higher nucleotides of purines and pyrimidines occur in the free state, e.g., ATP, ADP. Their third and second phosphate bonds can release about 8 kcal or more of free energy per mol on hydrolysis. This far exceeds the energy released on hydrolysis of most other covalent bonds. Therefore, these phosphate bonds of higher nucleotides are called High-Energy Bonds.
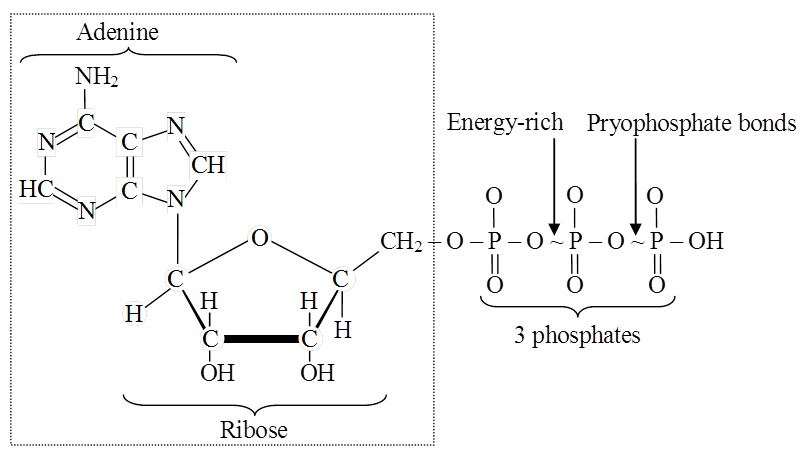
Nucleotides of the vitamins nicotinamide and riboflavin occur either freely or in combination with specific proteins, thus work as coenzymes. They do not participate in the formation of nucleic acids. Instead, they act along with oxidising enzymes and participate in oxidation reactions occurring in the cell.
Functions of Nucleotides
Purine and pyrimidine nucleotides polymerise to form nucleic acids.
Higher purine and pyrimidine nucleotides, particularly ATP, store energy in their high-energy phosphate bonds.
They are formed during photosynthesis and respiration.
Hydrolysis of the phosphate bonds of ATP releases their bond energy for driving energy-dependent reactions and processes.
Nicotinamide and riboflavin nucleotides act as coenzymes of oxidising enzymes.
Lipids
Lipids
Lipids are organic molecules containing hydrogen, carbon, and oxygen atoms that provide the structural and functional foundation for living cells. Because water is a polar molecule, these organic compounds are nonpolar molecules that are only soluble in nonpolar solvents and insoluble in water. These molecules are produced in the human liver and can be found in the oil, butter, whole milk, cheese, fried foods, and some red meats.
Structure of Lipids
Lipids are fatty acid polymers with a long, non-polar hydrocarbon chain and a short polar area that contains oxygen.Lipids are often insoluble in water. It's possible that they're just simple fatty acids. A carboxyl group is connected to the R group in a fatty acid. The R group could be methyl (–CH3), ethyl (–C2H5), or a combination of the two (1 carbon to 19 carbons). Palmitic acid, for example, comprises 16 carbons, including the carboxyl carbon. Arachidonic acid, including carboxyl carbon, has 20 carbon atoms.Saturated fatty acids have no double bonds, while unsaturated fatty acids have one or more C=C double bonds. Glycerol, which is trihydroxy propane, is another simple lipid. Glycerol and fatty acids are found in many lipids. The fatty acids are esterified with glycerol. Monoglycerides, diglycerides, and triglycerides are the three types of lipids. Based on their melting point, these are also known as fats and oils. Oils (e.g., gingely oil) have a lower melting point and hence remain as oil in the winter. Phosphorus and a phosphorylated organic molecule are found in some lipids. Phospholipids are what they're called. They're located in the membranes of cells. One example is lecithin. Lipids with more complicated structures are found in some tissues, particularly brain tissues.
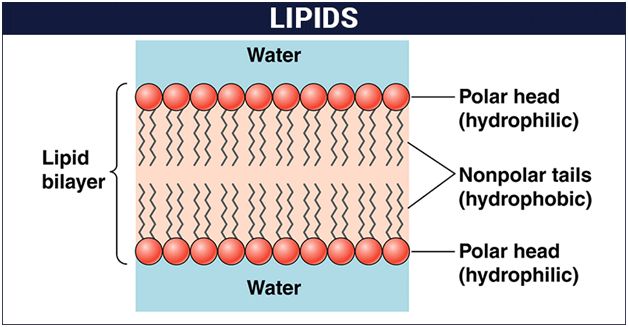
Listed below are some important characteristics of Lipids.
1. Lipids are oily or greasy nonpolar molecules, stored in the adipose tissue of the body.
2. Lipids are a heterogeneous group of compounds, mainly composed of hydrocarbon chains.
3. Lipids are energy-rich organic molecules, which provide energy for different life processes.
4. Lipids are a class of compounds characterized by their solubility in nonpolar solvents and insolubility in water.
5. Lipids are significant in biological systems as they form a mechanical barrier dividing a cell from the external environment known as the cell membrane.
Classification of Lipids:
Lipids serve a critical role in our bodies. They are a component of the cell membrane's structure. They aid in the production of hormones and provide energy to our bodies. They aid in appropriate meal digestion and absorption. If we eat them in the right amounts, they constitute a nutritious element of our diet. They play a vital function in signaling as well.
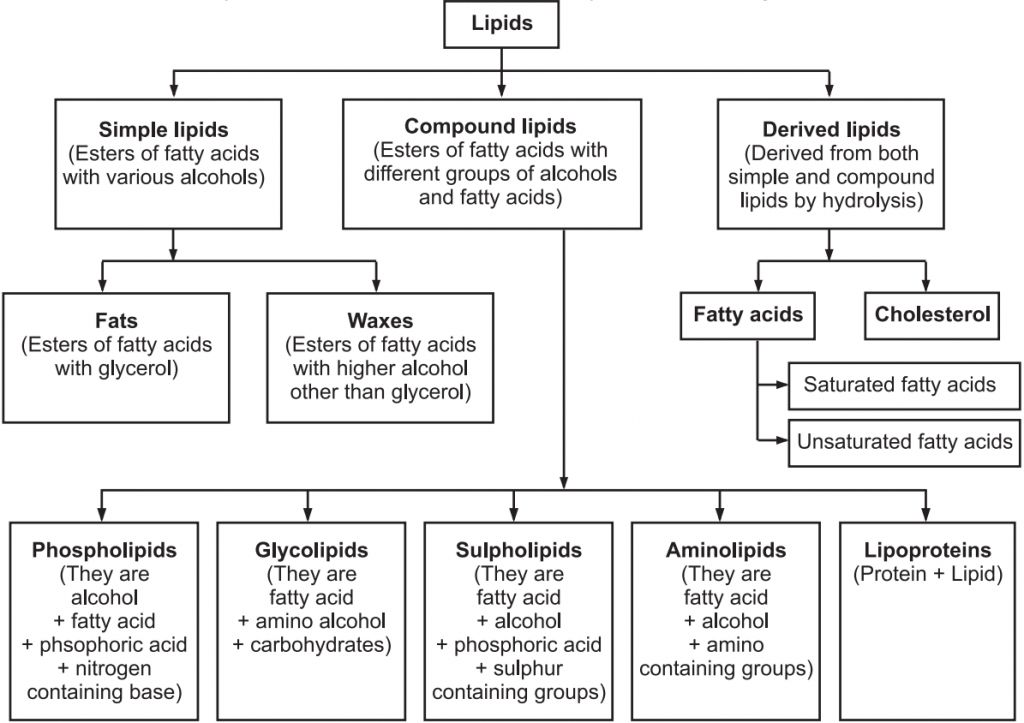
Nucleic Acids
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
NUCLEIC ACIDS
First discovered by Meischer.
Nucleic acids are giant molecules having a variety of functions.
There are two major types of nucleic acids Deoxyribonucleic Acid or DNA and Ribonucleic Acid or RNA.
DNA is found mainly in the nucleus but also occurs in chloroplasts and mitochondria.
It is the genetic material and contains all information needed for the development and existence of an organism.
RNA occurs as genetic material in some viruses.
Nucleic acids are linear polymers of purine and pyrimidine nucleotides.
The nucleotides are linked serially by phosphate groups, each linking the C5(5 – C) and the C'3(3' – C) of the pentoses of the successive nucleotides.
The DNA molecule consists of a double chain of nucleotides, whereas RNA consists of a single chain.
The nucleotides of DNA contain the bases adenine (A), thymine (T), guanine (G) and cytosine (C), while RNA contains A, G, C and uracil (U) instead of T.
The backbone of the nucleic acid is uniformly made up of alternating pentose and phosphate groups.
The pentose in DNA is deoxyribose (CsH10O4) and that in RNA is ribose (C5H10O5).
In the double stranded DNA, the bases of the opposite strands pair in a specific relationship by means of hydrogen bonds.
'A' always pairs with 'T' and 'G' always pairs with 'C'.
This complementarity is known as the Base-Pairing Rule.
Nucleic acids exhibit a wide variety of secondary structures. For example, one of the secondary structures exhibited by DNA is the famous Watson-Crick model.
This model says that DNA exists as a double helix.
The two strands of polynucleotides are antiparallel i.e. run in the opposite direction.
The backbone is formed by the sugar-phosphatesugar chain.
The nitrogen bases are projected more or less perpendicular to this backbone but face inside.
A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand.
There are two hydrogen bonds between A and T.
There are three hydrogen bonds between G and C.
Each strand appears like a helical staircase.
Each step of ascent is represented by a pair of bases.
At each step of ascent, the strand turns 36°.
One full turn of the helical strand would involve ten steps or ten base pairs.
Attempt drawing a line diagram.
The pitch would be 34 Å.
The rise per base pair would be 3.4 Å.
This form of DNA with the above mentioned salient features is called B-DNA.
In higher classes, you will be told that there are more than a dozen forms of DNA named after English alphabets with unique structural features.
In 1950, Erwin Chargaff found that in any DNA molecule:
(i) The amounts of purines and pyrimidines are equal i.e., A + G = T + C.
(ii) The amount of adenine is always equal to that of thymine; and the amount of guanine is always equal to that of cytosine (i.e., A =T and G =C).
(iii) The base ratio (A + T)/(G + C) may vary from one species to another, but is constant for a given species.
(iv) The deoxyribose sugar and phosphate components occur in equal proportions.
RNA is usually single-stranded, but sometimes (as in Reovirus and Rice dwarf virus), it is double-stranded.
RNA does not follow Chargaff's rules i.e., 1 : 1 ratio does not exist between purines and pyrimidines bases due to single-stranded nature and lack of complementarity.
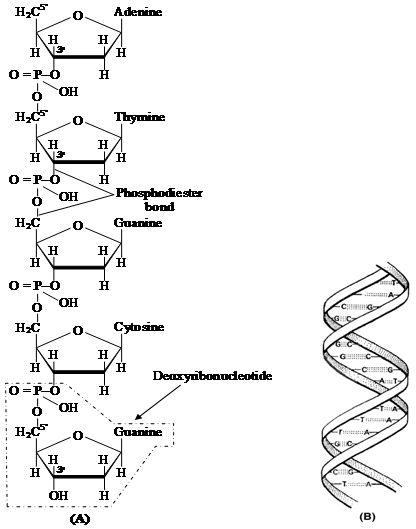
(B) Watson-Crick model of DNA double helix
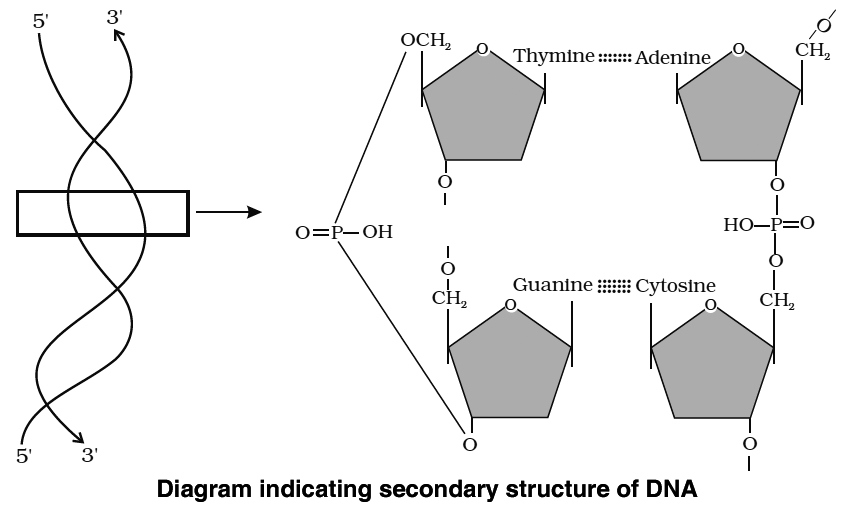
There are three types of non-genetic RNA.
(i) Messenger RNA (m-RNA) : It is produced in the nucleus and carries the information for the synthesis of proteins; it was discovered by Jacob and Monod (1961).
(ii) Ribosomal RNA (r-RNA) : It is the largest RNA and constitutes about 80% of total cellular RNA. Found in the ribosomes where protein synthesis takes place.
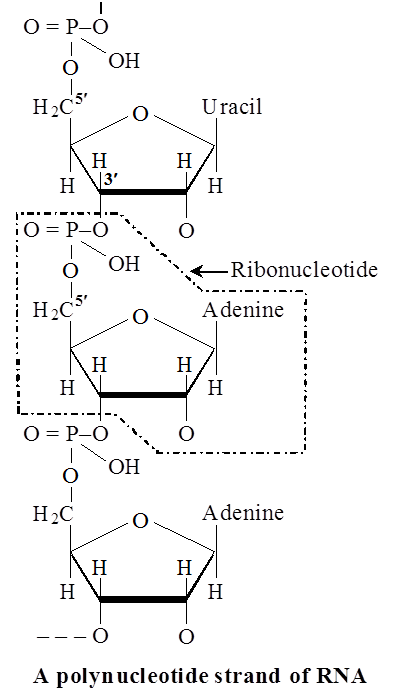
(iii) Transfer RNA or Soluble RNA or adaptive RNA (s-RNA, t-RNA) : It is the smallest type of RNA and constitutes about 10-15% of total cellular RNA. These are found in the cytoplasm and are different types (as many types as the types of amino acids -usually 20). Their function is to collect amino acids from the cytoplasm for protein synthesis.
t-RNA molecule is folded to form a clover leaf -like structure. This structure was given by Holley.
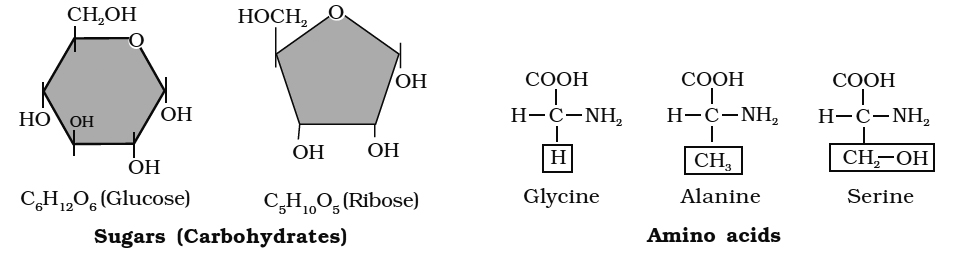
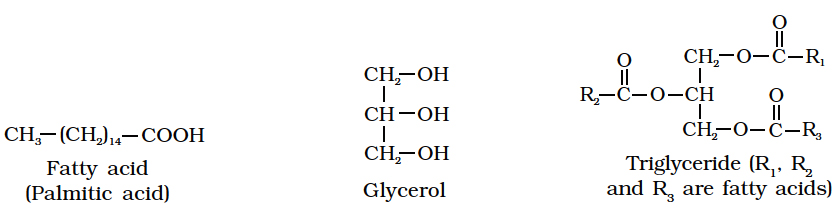
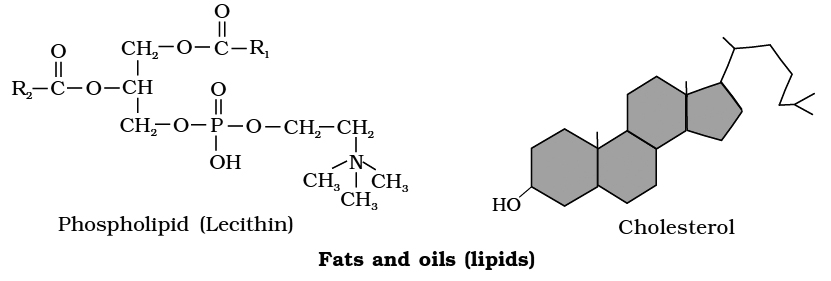
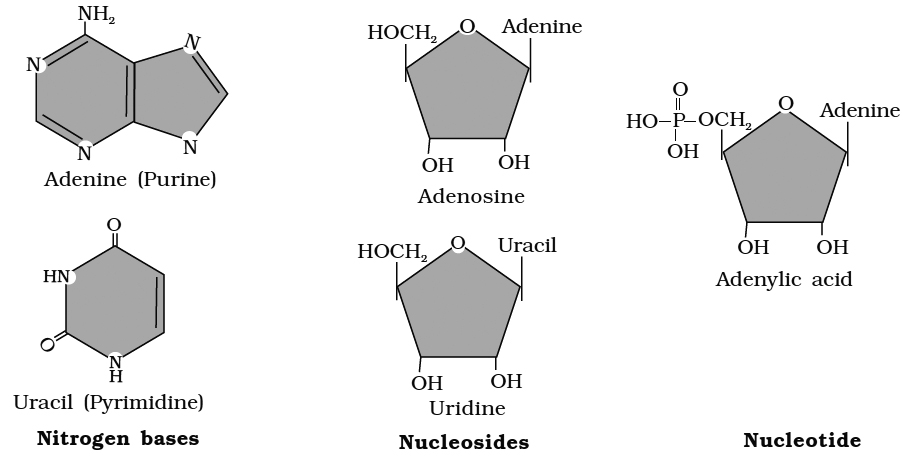
weight organic compounds in living tissues
Points to Remember
1. Study of X-ray diffraction patterns of DNAs isolated from various organisms by Wilkins, Franklin and Astbury revealed that DNA has a right handed helical structure.
2. Using all the available chemical and physical information, James Watson and F.C. Crick of Cambridge gave the double helix model of DNA for which they were awarded the Nobel Prize in 1962.
3. The width between the two backbones is constant and equal to the width of a base pair (i.e., width of a purine + a pyrimidine).
4. Along the axis of the molecule, the base pairs are 'spaced at intervals of 0.34 nm. Therefore, one complete turn of the double helix comprises 3.4 nm (10 base pairs).
5. There is no restriction on the sequence of bases in one chain. However, due to rule of base pairing, the sequence of one chain determines the sequence in the other. The two chains are thus said to be complementary. As a result, the (purine) adenine in either chain is associated with (pyrimidine) thymine in the other. Similarly, the (purine) guanine in either chain is associated with the (pyrimidine) cytosine in the other.
6. The two chains are held together by hydrogen bonding between the bases (joined together in pairs)-a single base from one chain being hydrogen-bonded to the complementary base from the complementary chain.
7. Adenine-thymine pair has two hydrogen bonds and the guanine–cytosine pair has three hydrogen bonds.
8. The double helix has a diameter of 20Å i.e., the distance between two strands is 19.8Å (or 20Å).
9. DNA with higher percentage of G C have more density than those with higher percentage of A = T.
10. Upon heating at temperatures above 80-90º, the two strands of DNA uncoil and separate (denaturation). On cooling, the strands come closer and are rejoined together (renaturation/ annealing). Low melting area of DNA is A = T base pairs.
11. 1 µm of DNA contains about 3000 base pairs.
12. The DNA is mostly right handed. This type of DNA exists in 4 forms -
(a) B form - the usual DNA, having 10 base pairs per turn.
(b) A form - having 11 base pairs (instead of 10 base pairs per turn), the base pairs are not perpendicular to the axis, but are tilted.
(c) C form - like B form, but having 9 base pairs per turn.
(d) D form - like B form, but have 8 base pairs per turn.
13. DNA with left handed coiling is called Z-DNA. In it, repeating unit is dinucleotide.
14. In some cases, as in × 174 and S-13 viruses, the DNA is single stranded.
Palindromic and repetitive DNA: DNA duplex possessing areas of same sequences of nucleotides is called palindromic DNA. Repetitive DNA has sequence of nitrogen bases repeated several times in tandem.
Nucleic Acids
Nucleic Acids
The nucleic acid is another form of macromolecule found in the acid-insoluble portion of any living tissue. Polynucleotides are a type of nucleotide. They make up the real macromolecular fraction of any living tissue or cell, together with polysaccharides and polypeptides. A nucleotide is the building block of nucleic acids. A nucleotide is made up of three chemically different parts. A heterocyclic molecule is one, a monosaccharide is another, and phosphoric acid or phosphate is the third. The nitrogenous bases adenine, guanine, uracil, cytosine, and thymine are heterocyclic molecules in nucleic acids. The purines Adenine and Guanine are substituted, with the pyrimidines Thymine and Cytosine. Purine and pyrimidine are the components for the skeletal heterocyclic ring.Polynucleotides include either ribose (a monosaccharide pentose) or 2' deoxyribose as a sugar. Deoxyribonucleic acid (DNA) is a nucleic acid that contains deoxyribose, whereas ribonucleic acid (RNA) contains ribose sugar.
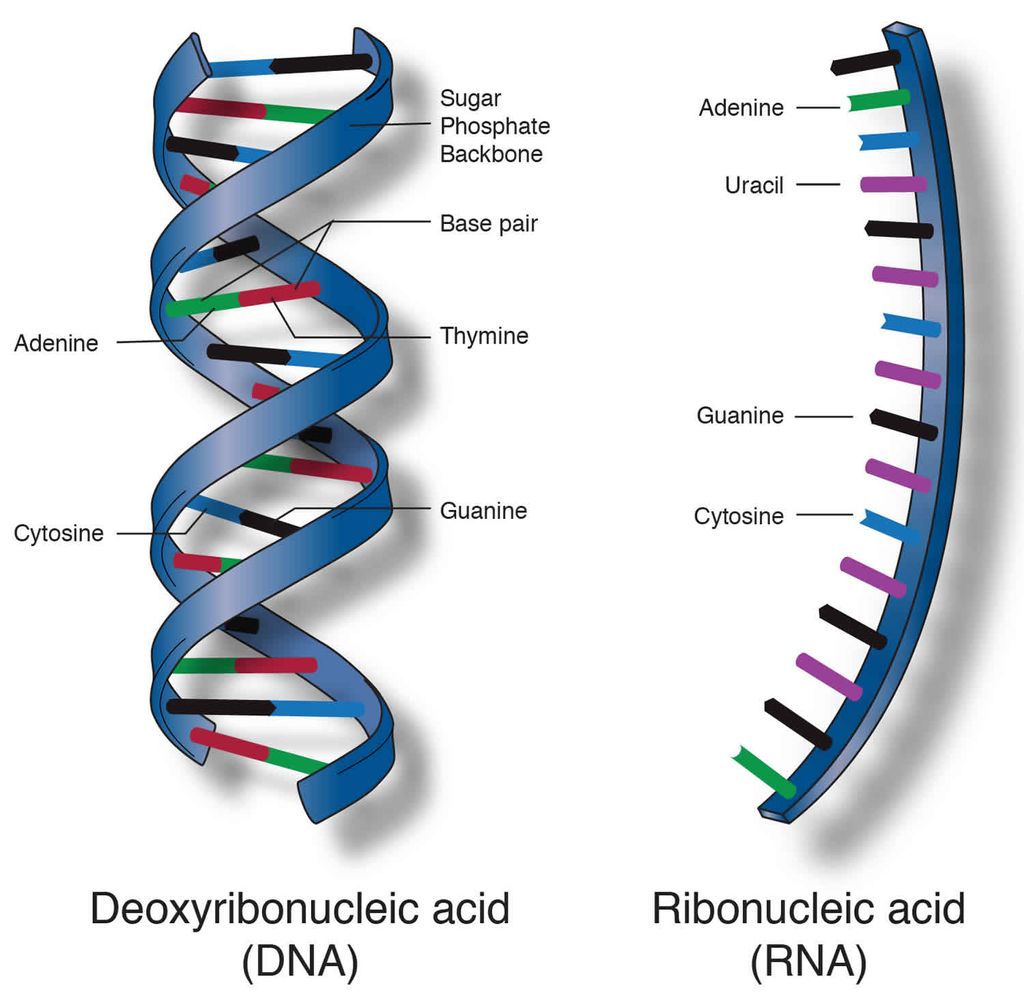
Secondary structures in nucleic acids come in a range of shapes and sizes. The famous Watson - Crick Model, for example, is one of DNA's secondary structures. According to this idea, DNA is a double helix. Polynucleotide strands are antiparallel, meaning they run in opposite directions. The sugar-phosphate-sugar chain makes up the backbone. The nitrogen bases are positioned perpendicular to the backbone but on the inside. One strand's A and G must base pair with the other strand's T and C, respectively. Between A and T, there are two hydrogen bonds, while between G and C, there are three hydrogen bonds.
Each thread resembles a spiral staircase. A pair of bases represent each stage of the ascent. The strand rotates 36 degrees with each climbing step. Ten steps or ten base pairs would be required to complete a full turn of the helical strand. It would be a 34 pitch. The increase would be 3.4 per base pair. B-DNA is a type of DNA that has the characteristics listed above. More than a dozen kinds of DNA named after English alphabets with unique structural properties are known to exist.
Concept of Metabolism and living state
Concept of metabolism and living state
Thousands of organic chemicals can be found in living species, whether they be bacteria, protozoa, plants, or animals. These substances or biomolecules exist at specific concentrations (mols/cell, mols/litre, and so on). The discovery that all biomolecules have a turnover was one of the most significant discoveries ever made. This means they're continually changing into and out of different biomolecules. Chemical reactions occur frequently in living organisms, breaking and making this possible. Metabolism refers to the sum of all these chemical events. The change of biomolecules occurs in each of the metabolic processes.
Removal of carbon di oxide from amino acids, resulting in an amine, removal of amino group in a nucleotide base, hydrolysis of a glycosidic bond in a disaccharide, and so on are some examples of metabolic transformations. There are tens of thousands of examples like this. The majority of these metabolic events are always related to other reactions and do not occur in isolation. In other words, metabolites are changed into one another through metabolic pathways, which are a set of related reactions. These metabolic pathways are comparable to city traffic in terms of complexity. There are two types of pathways: linear and circular. There are traffic crossroads where these paths cross one other.Like car traffic, the flow of metabolites through the metabolic pathway has a set rate and direction. The dynamic state of bodily constituents refers to this metabolite flux. What matters most is that this interconnected metabolic traffic runs smoothly and without a single reported hiccup under normal circumstances. Every chemical reaction in these metabolic pathways is a catalysed reaction, which is another distinguishing trait. In biological systems, there is no metabolic conversion that is not catalysed. Even the physical process of CO2 dissolving in water is a catalysed reaction in biological systems. Proteins are also catalysts that speed up the tempo of a metabolic conversation. Enzymes are the name given to these catalytic proteins.
Metabolic basis for living
Metabolic pathways can either lead to a more complex structure from a simpler structure (e.g., acetic acid becomes cholesterol) or a simpler structure from a complex structure (e.g., acetic acid becomes cholesterol) (for example, glucose becomes lactic acid in our skeletal muscle). The former are referred to as biosynthetic or anabolic pathways. The latter are referred to as catabolic pathways since they involve degradation. Anabolic pathways, use a lot of energy. Energy is required to assemble a protein from amino acids. Catabolic pathways, on the other hand, result in the release of energy. When glucose is metabolized to lactic acid in skeletal muscles, for example, energy is released. Glycolysis is a metabolic mechanism that takes glucose and converts it to lactic acid in ten stages.
Living organisms have figured out how to capture the energy released during breakdown and store it as chemical bonds. This bond energy is used as needed for biosynthetic, osmotic, and mechanical tasks that we do. The bond energy in a molecule called adenosine triphosphate is the most essential kind of energy currency in living systems (ATP).
The living state
Tens and thousands of chemical compounds in a living organism, otherwise called metabolites, or biomolecules, are present at concentrations characteristic of each of them. For example, the blood concentration of glucose in a normal healthy individual is 4.5-5.0 mM. The most important fact of biological systems is that all living organisms exist in a steady-state characterized by concentrations of each of these biomolecules. These biomolecules are in metabolic flux. Any chemical or physical process moves spontaneously to equilibrium. The steady state is a non-equilibrium state. One should remember from physics that systems at equilibrium cannot perform work. As living organisms work continuously, they cannot afford to reach equilibrium.
Thus, to be able to conduct work, the living state is a non-equilibrium steady-state; the living process is a constant endeavour to avoid slipping into equilibrium. This is accomplished by the use of energy. Metabolism is the process by which energy is produced. As a result, the terms "living state" and "metabolism" are interchangeable. There can be no living condition without metabolism.
Enzymes
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
ENZYMES
Enzymes are proteinaceous, biocatalysts.
First enzyme discovered by Buchner.
Term enzyme was given by Kuhne.
Zymase (from yeast) was the first discovered enzyme. (Buchner)
The first purified and crystalized enzyme was urease (by J.B. Sumner) from Canavalia/Jack Bean (Lobia plant).
Proteinaceous nature of enzyme was established by Northrop and Sumner.
DEFINITION
Enzymes are biocatalysts made up of proteins (except ribozyme), which increases the rate of biochemical reactions by lowering down the activation energy.
First discovered ribozyme was L19 RNAase by T.Cech from rRNA of a protozoan Tetrahymena thermophila and RNAase P or Ribionuclease P by Altman in prokaryotic cell (Nobel prize).
GENERAL PROPERTIES OF ENZYMES
Large sized biomolecules, colloid nature with high molecular weight.
Large size (equal to colloid particles) provide, more surface area so passes large no. of active site. Large number of substrate converted into product by one molecule of enzyme at a time.
Highest molecular weight is of enzyme pyruvate dehydrogenase complex (46 lakh) participate in link reaction of respiration.
Proteinous nature
Monomer unit of a enzyme is Amino acid.
Amino acids linked togather to form polypeptide chain.
Enzymes are polypeptide chains.
Most of enzymes arrange in tertiary structure of protein or globular proteins except isoenzyme (Quaternary st.).
Tertiary structure of protein provides stability and water soluble nature to enzymes.
Synthesis of enzymes occurs on ribosomes under the control of genes.
According to one gene one polypeptide hypothesis, if a enzyme is made up of same kind of polypeptide chains then synthesize under control of same gene and if made up of different kinds of polypeptide chains then synthesized under the control of different genes. e.g., Rubisco, cytochrome, oxidase, Nitrogenase.
Specificity
Enzymes are specific for pH, temperature and substrate.
pH - The common pH range of enzymes activity is 6 - 8.
Every enzyme works on specific pH, Pepsin-2.5 pH, Hydrolase-4-5.
Rubisco, Pepcase-8.5 pH, Trypsin - 8.5 pH.
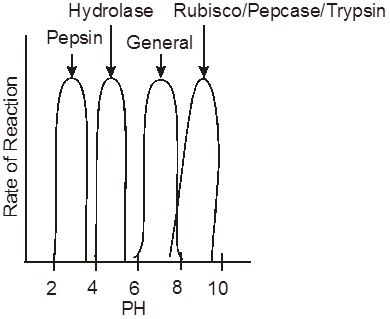
Temperature
Common range of temperature for enzyme activity is 20º – 40°C.
Enzymes works on body temperature of organism not on environmental temperature.
Enzymes of plants are affected by evironmental temperature change as plants does not show homeostasis.
At low temperature enzymes become functionally inactive, at high temperature denatured.
Substrate
Every enzyme works on specific substrate.
Substrate binds at active site of enzyme which is made of specific sequence of amino acids and recognise it’s substrate.
Ex. succinic dehydrogenase acts on succinic acid while pyruvate dehydrogenase acts on pyruvic acid.
Enzymes increase the rate of reaction by decreasing activation energy.
Activation Energy - Minimum amount of energy more than the free energy of reactents required to reach the transiation state of chemical reaction or to undergo the chemical reaction.
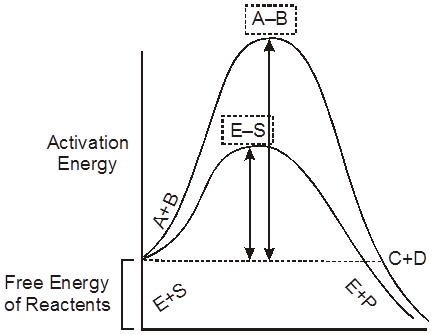
Turn Over Nubmer (T.O.N.) -
The number of reactent moleules converted into product by one molecule of enzyme in unit time
Highest T.O.N is of carbonic anhydrase (360 lakh / minute)
CO2 + H2O  H2CO3
H2CO3
KM constant -
Enymes follows the Michaelis-Menten reaction kinetics.
It represents the substrate concentration at which rate of enzymetic reaction becomes the half of maximum velocity or rate.
If a enzyme passes high km constant then it’s affinity towards substrate is low and rate reaction is also low.
The energy required for a chemical reaction to proceed is called Activation energy.
The enzymes lower the activation energy. (Remember that enzymes cannot start the chemical reaction)
With the increase in concentration of substrate the enzymatic velocity also increases. At a certain value all the active site of the enzyme-molecules are saturated and the increase in substrate concentration does not increase the velocity of the enzymatic reaction.
(The concentration of substrate at which the velocity of enzymatic action reaches half of its maximum value, is called Km value or Michaelis constant).
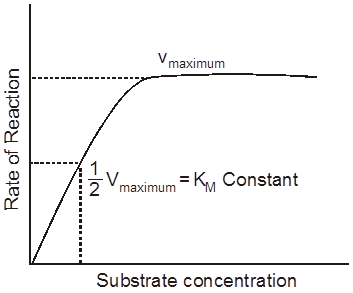
Higher is the affinity of an enzyme for a substrate the lower is its Km value, i.e.
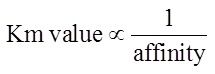
Ki constant (Enzyme inhibitor complex dissociation constant)
The substrate concentration at which enzyme inhibitor complex dissociate and reaction becomes normal
It is applicable only for competitive reversible inhibitions.
STRUCTURE OF ENZYME
Simple enzymes
They are made up of only protein. eg. pepsin, trypsin.
Conjugated enzymes
They are made up of protein & non protein part.
Co-enzymes - Co-enzymes are non-protein, orgainc groups, which are loosely attached to apoenzymes. They are generally made up to vitamins.
Prosthetic group - When non-protein part is tightly or firmly attached to apoenzymes.
Metal activators/co-factros/metallic factor :- Lossely attached inorganic co-factor eg. Mn, Fe, Co, Zn, Ca, Mg, Cu
Active site :
The part of polypeptide chain made up of specific sequence of amino acids at which specific substrate is to be binded and catalysed, known as active site. Very specific sequence of amino acids, at active site is determined by genetic codes.
Allosteric site :
Besides the active site's some enzymes posess additional sites, at which chemical other than substrate (allosteric modulators) are bind. These sites are known as allosteric sites and enzyme with allosteric sites are called as allosteric enzymes. e.g. hexokinase, phosphofructokinase.
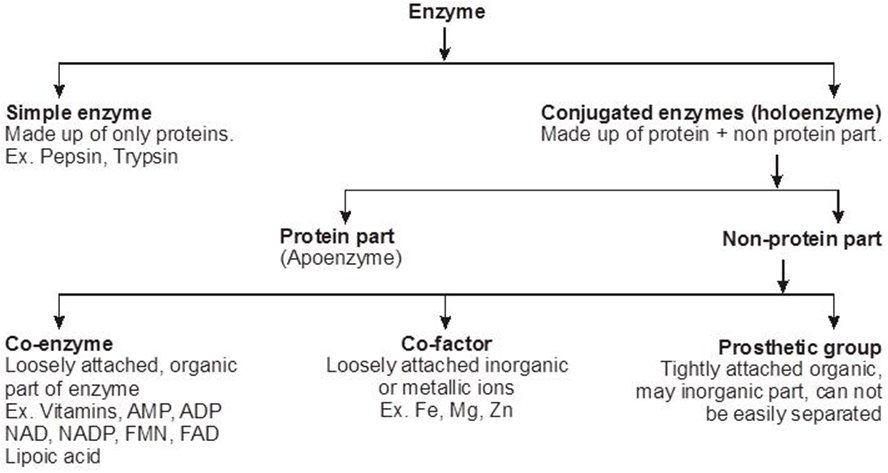
Concept Builder
TERMINOLOGY
Endoenzymes - Enzymes which are functional only inside the cells. Ex. Enzymes of metabolism.
Exoenzymes - Enzymes catalysed the reactions outside the cell Eg:- enzymes of digestion, some enzymes of insectivorous plants, Zymase complex of fermentation.
Proenzyme/Zymogen - These are precursor of enzymes or inactive forms of enzymes.eg. Pepsinogen, Trypsinogen etc.
Isoenzymes - Enzymes having similar action, but little difference in their molecular configuration are called isoenzymes. 16 forms of -amylase of wheat & 5 forms of LDH (Lactate dehydrogenase) 3 Forms of Pepcase are known. These all isoenzyme forms are synthesised by different genes and tissue and organ specific.
Inducible enzymes - When formation of enzyme is induced by substrate availability. e.g. Lactase, Nitrogenase, -galactosidase.
Extremozymes - Enzymes, which may also function at extremely adverse conditions (very high temperature) e.g. Taq polymerase.
Abzymes - When the monoclonal antibodies (Mab) are used as enzymes.
Biodetergents - Enzymes used in washing powders are known as bio-detergents e. g.-amylase, lipase, proteolytic enzymes.
House keeping/constitutive enzymes - Which are always present in constant amount & are also essential to cell. Ex. Enzymes of cell respiration.
Concept Builder
Metal ion Metalloenzyme
Fe++, Fe+++ Cytochrome oxidase, catalase, aconitase, peroxidase
Ca++ Lipase, Succinic dehydrogenase, thrombin, thrombokinase
Mg++ Hexokinase, pyruvate kinase, DNA Polymerase, enolase, phosphotransferase
Cu++ Cytochrome oxidase, tyrosinase
Co++ Ascorbic acid oxidase, Peptidases
Mo Dinitrogenase, nitrate reductase
Mn++ Ribouncieotide reductase, Arginase
Zn++ Alcohol dehydrogenase, Carbonic anydrase, LDH, carboxypeptidase,
Glycine reductase, thiolase
Se Glycine reductase, thiolase
K+ Pyruvate kinase
Ni Urease
Cl– Salivary amylase
Na+ ATPase
Classification of Enzymes
Enzymes were variously named in the past.
Enzyme names such as ptyalin (salivary amylase), pepsin and trypsin, give no indication of their action.
Other enzymes such as amylase, sucrase, protease and lipase were named after the substrates on which they act-amylose (starch), sucrose, protein and lipids respectively.
Still others were named according to the source from which they were obtained-papain from papaya, bromelain from pineapple (belongs to the family Bromeliaceae).
Some like DNA polymerase indicate its specific action, polymerisation.
The Duclaux (1883) provided a system for naming enzymes by adding suffix -ase at the end of enzyme name.
In this system, each enzyme ends with an -ase and consists of two parts, the first part indicates its substrate and the second the reaction catalysed.
For example, glutamate pyruvate transaminase transfers an amino group from the substrate glutamate to another substrate pyruvate.
However, arbitrary names like ptyalin and trypsin still continue to be used because of their familiarity.
Enzymes are grouped into six major classes:
Class 1. Oxidoreductases:
These catalyse oxidation or reduction of their substrates and act by removing or adding electrons (and/or H+) from or to substrates e.g., cytochrome oxidase oxidises cytochrome.
Class 2. Transferases:
These transfer specific groups from one substrate to another. The chemical group transferred in the process is not in a free state, e.g., glutamate pyruvate transaminase.
Class 3. Hydrolases:
These break down large molecules into smaller ones by the introduction of water (hydrolysis) and breaking of specific covalent bonds. Most digestive enzymes belong to this category, e.g., amylase which hydrolyses starch, lipases.
Class 4. Lyases:
These catalyse the cleavage of specific covalent bonds and removal of groups without hydrolysis, e.g., histidine decarboxylase cleaves C-C bond in histidine to form carbon dioxide and histamine.
Class 5. Isomerases:
These catalyse the rearrangement of molecular structure to form isomers, e.g., phosphohexose isomerase changes glucose-6-phosphate to fructose-6-phosphate (both are hexose phosphates).
Class 6. Ligases:
These catalyse covalent bonding of two substrates to form a large molecule. The energy for the reaction is derived from the hydrolysis of ATP. Pyruvate carboxylase combines pyruvate and carbon dioxide to form oxaloacetate at the expense of ATP.
Factors Affecting Enzyme Activity
Tile activity of an enzyme can be affected by a change in the conditions which can alter the tertiary structure of the protein.
These include temperature, pH, change in substrate concentration or binding of specific chemicals that regulate its activity.
Temperature and pH
Enzymes generally function in a narrow range of temperature and pH (in figure).
Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH.
Activity declines both below and above the optimum value.
Low temperature preserves the enzyme in a temporarily inactive state, whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
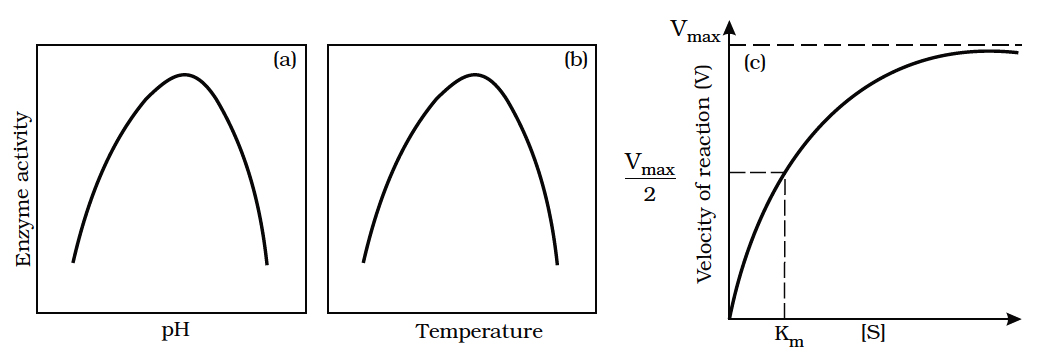
Effect of change in : (a) pH, (b) Temperature and
(c) Concentration of substrate on enzyme activity
(i) Optimum Temperature:
Enzymes generally work over a narrow range of temperatures.
Usually it corresponds to the body temperature of the organism.
For instance, human enzymes work at the normal body temperature.
Each enzyme shows its highest activity at a particular temperature called the optimum temperature.
Activity declines both above and below the optimum temperature.
Temperature:
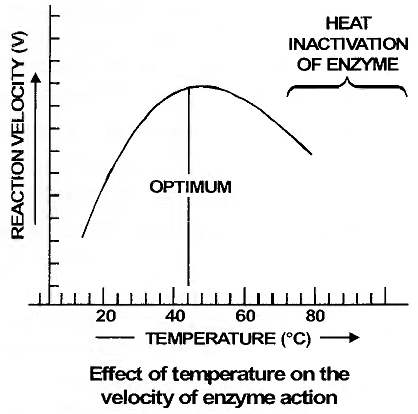
Every enzyme has a specific optimum temperature.
According to the general rule of thumb the Q10 (temperature coefficient) for enzymes is 2-3, i.e., in between minimum and optimum temperature (5-40°C), the rate of reaction increases 2-3 times with rise in 10°C temperature.
If temperature is reduced to near or below freezing point, the enzymes are inactivated (not denatured).
Most enzymes show maximum activity in a temperature range of 25-40°C.
Enzymes are thermolabile i.e., are denatured at high temperature.
The loss of catalytic properties begins at 35ºC and is almost complete around 60°C.
However, dried enzyme extracts can endure temperature of 100°C-120°C or even higher.
That is why, dry seeds can endure higher temperature than germinating seeds.
Thermal stability is thus an important quality of some enzymes isolated from thermophilic organisms.
(ii) Optimum pH :
Each enzyme shows its highest activity at a specific pH.
This is called the optimum pH.
Activity declines both above and below the optimum pH.
Most intracellular enzymes function best around neutral pH.
Some digestive enzymes have their optimum in the acidic or alkaline range.
For example, the protein digesting enzyme pepsin found in the stomach, has an optimum pH of 2.0.
Another protein-digesting enzyme, trypsin, found in the duodenum, functions best in an alkaline pH 8.0.
(iii) Concentration of Substrate:
With the increase in substrate concentration, the velocity of the enzymatic reaction rises at first.
The reaction ultimately reaches a maximum velocity (Vmax) which is not exceeded by any further rise in concentration of the substrate.
This is because the enzyme molecules are fewer than the substrate molecules and after saturation of these molecules, there are no free enzyme molecules to, bind with the additional substrate molecules.
Characteristics of Enzymes
1. Proteinaceous nature: All enzymes are chemically made up of proteins (except ribozyme and ribonuclease-P). They, however may have additional inorganic or organic substances for their activity.
2. Amphoteric nature: The enzymes are capable of ionizing either as an acid or as a base depending upon the acidity of the external solution. Hence, their nature is amphoteric i.e., they can act as acid as well as base.
3. Colloidal nature: They are colloidal in nature due to which they present a large surface area for reaction to take place. They are hydrophilic and form hydrosol in the cell.
4. Reversibility: Like a true catalyst enzymes have been found to accelerate the chemical reaction in either direction i.e., forward and backward depending upon the availability of suitable energy requirement, pH, concentration of end products and availability ot reactants.

5. Molecular weight: Enzymatic proteins are substances of high molecular weight. Peroxidase, which is one of the smallest enzymes, has a molecular. weight of 40,000 whereas catalase, one of the largest enzymes, has a molecular weight of 250,000 (Urease 483,000).
6. Specificity of enzyme: Enzymes are highly specific in nature i.e. , a particular enzyme can catalyze only a particular type of reaction e.g., the enzyme malic dehydrogenase removes hydrogen atom from malic acid and not from other keto acids. The specificity of enzyme is determined by sequence of amino acids in the active sites. The active site possesses a particular binding site which complexes only with specific substrate. Thus a suitable substrate fulfils the requirements of active site and closely fixes with it.
7. Unchanged form: Enzymes are in no way transformed or used up in the chemical reaction but come out unchanged at the end of reaction.
8. Chemical reaction: Enzymes do not start a chemical reaction but increase the rate of chemical reaction. They do not change the equilibrium as well. However, they increase the rate of chemical reaction and bring about equilibrium very soon. Carbonic anhydrase is the fastest acting enzyme.

In absence of any enzyme this reaction is very slow, with about 200 molecules of H2C03 being formed in an hour. However, by using the enzyme present within the cytoplasm called carbonic anhydrase, the reaction speeds dramatically with about 600,000 molecules being formed every second. Hence, the enzyme accelerates the reaction rate by about 10 million times.
9. Efficiency: Efficiency of an enzyme is judged by its 'turn over number' i.e., number of substrate molecules changed per minute by a molecule of enzyme. It depends upon the number of active sites present over an enzyme, precise collisions between reactants and the rate of removal of end products The optimum turn-over number for enzyme carbonic anhydrase is 36 million, catalase 5 million, sucrase or invertase 10,000 and flavoprotein 50.
How Enzymes Speed Up Reactions?
A certain amount of energy is necessary to initiate any chemical reaction.
This is called activation energy or free energy of activation.
In a population of molecules of each substrate, the majority have average kinetic energy, some have higher and some lower than the average energy.
Under normal temperature, only the molecules having relatively high energy are likely to react to form the product.
Therefore, the reaction takes place very slowly.
One way to make the reaction go faster is to raise the temperature of the mixture.
Heat increases the kinetic energy of the molecules, causing their collisions and reaction.
The other method of quickening the reaction is by adding an enzyme.
The enzyme lowers the activation energy of the reaction and allows a large number of molecules to react at time.
Exactly how the enzymes lower the activation energy is not clear.
However, it is known that the enzymes combine with the substrate molecules and bring them close together which favours their collisions in the most suitable directions and locations for the reaction to occur.
The inorganic catalysts work in the same manner.
It is now held that conformational changes in the active sites of the enzymes actually "push" the substrate molecules toward an interaction.
Hydrolysis of starch into glucose is an organic chemical reaction.
Rate of a physical or chemical process refers to the amount of product formed per unit time.
Rate can also be called velocity if the direction is specified for a given reaction.
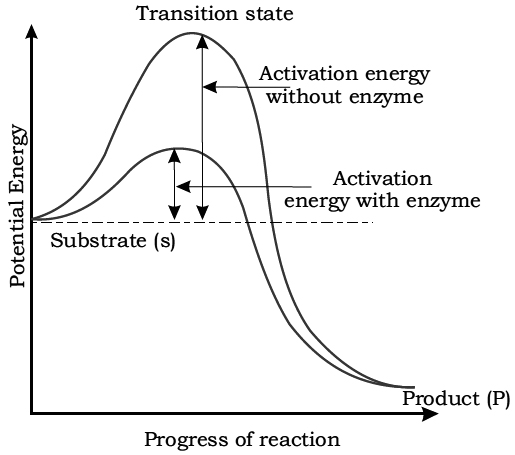
Activation energy requirement of uncatalysed and enzyme-catalysed reactions.
Reactants absorb energy from surroundings to climb the hill of activation energy (EA) and reach the unstable, short lived, transition state. Enzyme speeds up the reaction by reducing the uphill climb to the transition state. In transition state, the reactants are in an unstable condition and reaction can occur.
Concept Builder
1. Some laundry detergents contain enzymes. Generally proteases and amylases are added in detergents used for washing clothes and amylase in detergents used for dish washing. They help to break down proteins and other substances from food that may stain clothing.
2. Dairy digestive supplements contain lactase.
3. Baby foods: Trypsin is added to partially pre-digest the food.
4. Onions: Onions contain sulfur compounds called amino acid sulfoxides which result in the flow of lacrimal fluid.
Mode of Enzyme Action
1. Lock and key Hypothesis was put forward by Emil Fischer in 1894.
2. Induced Fit Theory was proposed by Koshland in 1959. According to this theory the active site of the enzyme contains two groups, buttressing and catalytic. The buttressing group is meant for supporting the substrate.
Mechanism of Enzyme Action
Two hypothesis have been put forward to explain the mode of enzyme action.
1. Lock and Key Hypothesis:
This hypothesis was given by Emil Fischer (1894).
According to this hypothesis, both enzyme and substrate molecules have specific geometrical shapes.
It is similar to the system of lock and key, which have special geometrical shapes in the region of their activity.
The active sites contain special groups having -NH2, -COOH, -SH for establishing contact with the substrate molecules.
Just as a lock can be opened by its specific key, a substrate molecule can be acted upon by a particular enzyme.
This also explains the specificity of enzyme action.
After coming in contact with the active site of the enzyme, the substrate molecules or reactants form a complex called enzymesubstrate complex.
In the enzyme substrate complex, the molecules of the substrate undergo chemical change and form products.
The product no longer fits into the active site and escapes in surrounding medium, leaving the active site free to receive more substrate molecules.
This theory explains how a small concentration of enzyme can act upon a large amount of the substrate.
It also explains how the enzyme remains unaffected at the end of chemical reaction.
The theory explains how a substance having a structure similar to the substrate can work as competitive inhibitor.
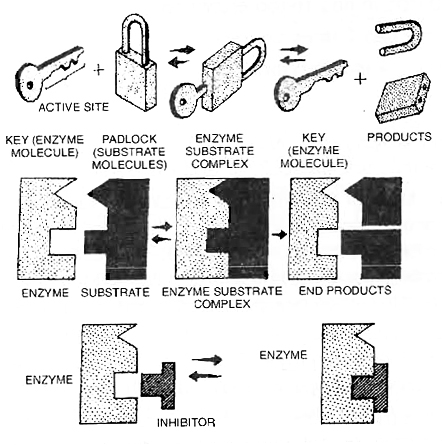
Lock and key hypothesis to show the specificity of enzymes
Enzyme + Substrate  Enzyme — Substrate Complex
Enzyme — Substrate Complex
Enzyme — Substrate Complex  Enzyme + End Products
Enzyme + End Products
2. Induced Fit Hypothesis:
This hypothesis was proposed by Koshland (1960).
According to this hypothesis the active site of the enzyme does not initially exist in a shape that is complementary to the substrate but is induced to assume the complementary shape as the substrate becomes bound to the enzyme.
According to Koshland, "the active site is induced to assume a complementary shape in much the same way as a hand induces a change in the shape of a glove."
An active site of an enzyme is a crevice or a pocket into which the substrate fits.
Thus, enzymes through their active site, catalyse reactions at a high rate.
Hence, according to this model, the enzyme (or its active site) is flexible.
Induced-fit theory of enzyme action. A. active site of enzyme. B. substrate molecule.
C. Enzyme-substrate complex with conformational changes so as to bring the
catalytic group against the substrate bonds to be broken
The active site of the enzyme contains two groups-
(a) Buttressing group is meant for supporting the substrate.
(b) Catalytic group is meant for catalysing the reaction.
When substrate comes in contact with the buttressing group, the active site changes to bring the catalytic group opposite the substrate bonds to be broken.
Iso-enzymes
Multiple molecular forms of an enzyme (synthesized by different genes) occurring in the same organism and having a similar substrate activity. Over 100 enzymes are known to have iso-enzymes such as
(i) -amylase of wheat endosperm has 16-iso-enzymes.
(ii) Lactic acid dehydrogenase has 5 iso-enzymes.
(iii) Alcohol dehydrogenase has 4 iso-enzymes.
Site of Enzyme Action
All enzymes are produced in the living cells.
About 2,000 enzymes have been recorded.
These are of two types with regard to the site where they act: intracellular and extracellular.
1. Intracellular Enzymes:
Most of the enzymes remain and function inside the cells.
They are called the intracellular enzymes or endoenzymes.
Some are dissolved in the cytoplasmic matrix.
A water extract of ground up liver cells contains all the eleven enzymes necessary to change glucose to lactic aid.
Certain enzymes are bound to particles, such as ribosomes, mitochondria and chloroplast.
The respiratory enzymes needed to convert lactic acid to carbon dioxide and water are found in the mitochondria.
2. Extraceliular Enzymes:
Certain enzymes leave the cells and function outside them.
They are called the extracellular enzymes or exoenzymes.
They mainly include the digestive enzymes, e.g., salivary amylase, gastric pepsin, pancreatic lipase; secreted by the cells of the salivary glands, gastric glands and pancreas, respectively.
Lysozyme present in tears and nasal secretion is also an exoenzyme.
The enzymes retain their catalytic action even after extraction from the cells.
Rennet tablets, containing the enzyme rennin from the calf's stomach, are used to coagulate milk protein caseinogen for cheese (casein) formation.
Inhibition of Enzyme Action
The activity of an enzyme is also sensitive to the presence of specific chemicals that bind to the enzyme.
When the binding of the chemical shuts off enzyme activity, the process is called inhibition and the chemical is called an inhibitor.
Following types of enzyme inhibition can occur :
(i) Competitive Inhibition
The action of an enzyme may be reduced or inhibited in the presence of a substance that closely resembles the substrate in molecular structure.
Such an inhibitor is called a Competitive Inhibitor of that enzyme.
Due to its close structural similarity with the substrate, the inhibitor competes with the latter for the substratebinding site of the enzyme.
Consequently, the enzyme cannot participate in catalysing the change of the substrate.
As a result the enzyme action declines, e.g., the inhibition of succinic dehydrogenase by malonate, which closely resembles succinate in structure.
This may be compared to a lock jammed by a key similar to the original key.
Such competitive inhibitors are often used in the control of bacterial pathogens.
For instance, sulpha drugs are competitive inhibitors of folic acid synthesis in bacteria as they substitute for p-amino benzoic acid, thus preventing the next step in the synthesis.
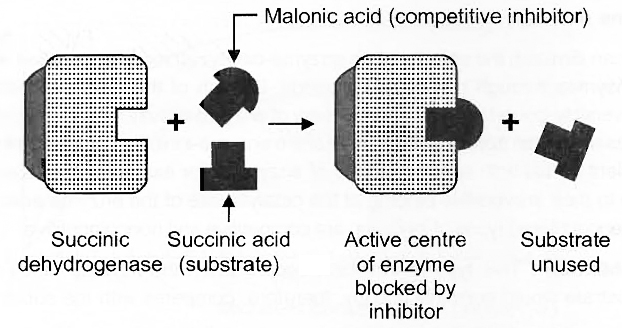
Competitive inhibition of enzyme action
(ii) Non-competitive Inhibition
Cyanide kills an animal by inhibiting cytochrome oxidase, a mitochondrial enzyme essential for cellular respiration.
This is an example of non-competitive inhibition of an enzyme.
Here the inhibitor (cyanide) has no structural similarity with the substrate (cytochrome c) and does not bind with the substrate-binding site but at some other site of the enzyme.
Thus, in non-competitive inhibition, substrate binding takes place but no products are formed.
(iii) Allosteric Modulation or Feedback Inhibition
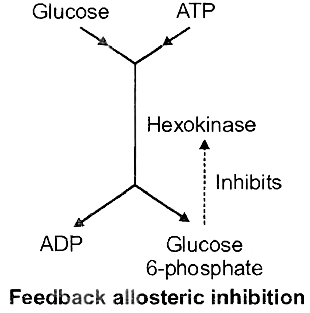
The activities of some enzymes, particularly those which form a part of a chain of reactions (metabolic pathway), are regulated internally.
Some specific low molecular weight substance, such as the product(s) of another enzyme further on in the chain, acts as the inhibitor.
Such a modulator substance binds with a specific site of the enzyme different from its substrate-binding site.
This binding increases or decreases the enzyme action. Such enzymes are called Allosteric Enzymes.
Examples :
(a) Hexokinase which changes glucose to glucose-6-phosphate in glycolysis. Decline in enzyme activity by the allosteric effect of the product is called Feedback Inhibition, e.g., allosteric inhibition of hexokinase by glucose-6-phosphate.
(b) Enzyme phosphofructokinase is activated by ADP and inhibited by ATP.
(c) Another example is inhibition of threonine deaminase by isoleucine. Amino acid isoleucine is formed in bacterium Escherichia coli in a 5-step reaction from threonine. When isoleucine accumulates beyond a threshold value, its further production stops.
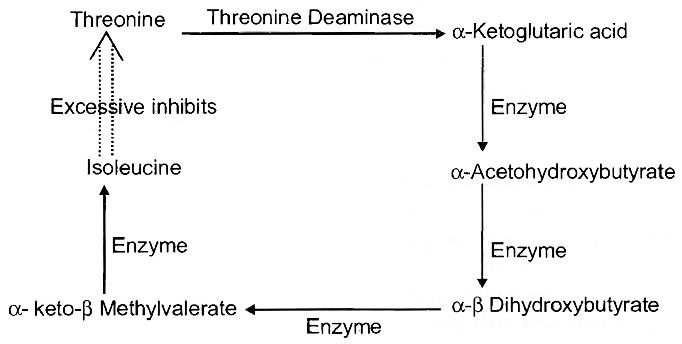
Inhibition of Enzyme Activity
Any substance that can diminish the velocity of an enzyme-catalyzed reaction is called an inhibitor.
Reversible inhibitors bind to enzymes through non-covalent bonds.
Dilution of the enzyme-inhibitor complex results in dissociation of the reversibly-bound inhibitor and recovery of enzyme activity.
Irreversible inhibition occurs when an inhibited enzyme does not regain activity upon dilution of the enzyme-inhibitor complex.
Some irreversible inhibitors act by forming covalent bonds with specific groups of enzymes; for example, the neurotoxic effects of certain insecticides are due to their irreversible binding at the catalytic site of the enzyme acetylcholinesterase.
The two most commonly encountered types of inhibition are competitive and noncompetitive.
A. Competitive inhibition :
This type of inhibition occurs when the inhibitor binds reversibly to the same site that the substrate would normally occupy, therefore, competes with the substrate for that site.
1. Effect on Vmax: The effect of a competitive inhibitor is reversed by increasing [S]. At a sufficiently high substrate concentration, the reaction velocity reaches the Vmax observed in the absence of inhibitor.
2. Effect on Km : A competitive inhibitor increases the apparent Km for a given substrate. This means that in the presence of a competitive inhibitor more substrate is needed to achieve 1/2 Vmax, e.g., sulpha drugs for folic acid synthesis in bacteria and inhibition of succinic dehydrogenase by Malonate.
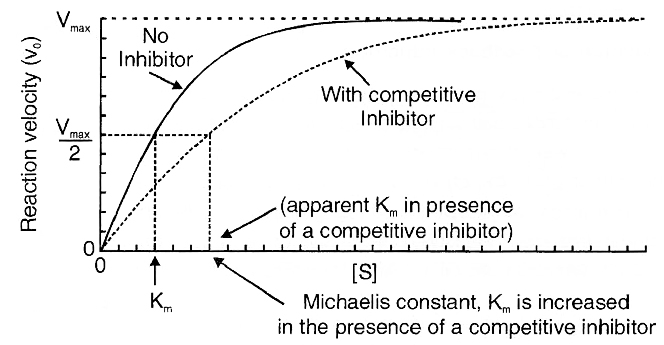
Effect of a competitive inhibitor on the reaction
velocity (v0) versus substrate [S] plot.
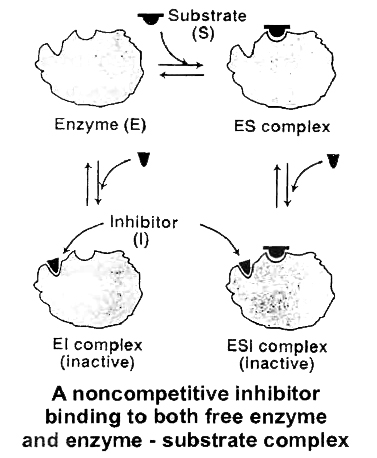
B. Non-competitive inhibition:
This type of inhibition is recognized by its characteristic effect on Vmax. Noncompetitive inhibition occurs when the inhibitor and substrate bind at different sites on the enzyme. The noncompetitive inhibitor can bind either free enzyme or the ES complex, thereby preventing the reaction from occurring.
1. Effect on Vmax : Noncompetitive inhibition cannot be overcome by increasing the concentration of substrate. Thus, noncompetitive inhibitors decrease the Vmax of the reaction.
2. Effect on Km : Noncompetitive inhibitors do not interfere with the binding of substrate to enzyme. Thus, the enzyme shows the same Km in the presence or absence of the noncompetitive inhibitor. e.g., cyanide kills an animal by inhibiting cytochrome oxidase.
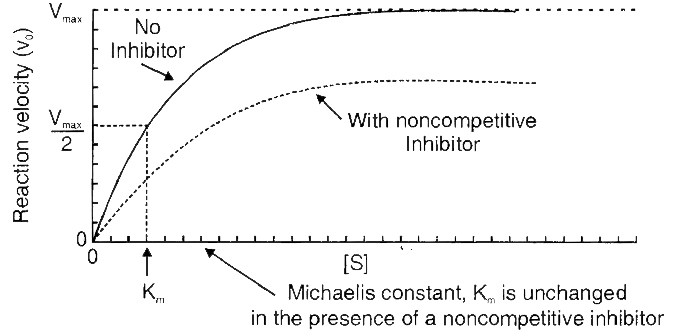
Effect of a noncompetitive inhibitor on the reaction
velocity (v0) versus substrate [S] plot.
Summary
Although there is a bewildering diversity of living organisms, their chemical composition and metabolic reactions appear to be remarkably similar.
The elemental composition of living tissues and non-living matter appears to be similar when analysed qualitatively.
However, a closer examination reveals that the relative abundance of carbon, hydrogen and oxygen is higher in living systems when compared to inanimate matter.
The most abundant chemical in living organisms is water.
There are thousands of small molecular weight (< 1000Da) biomolecules.
Amino acids, monosaccharide and disaccharide sugars, fatty acids, glycerol, nucleotides, nucleosides and nitrogen bases are some of the organic compounds present in living organisms.
There are 21 types of amino acids and 5 types of nucleotides.
Fats and oils are glycerides in which fatty acids are esterified to glycerol.
Phospholipids contain, in addition, a phosphorylated nitrogenous compound.
They are found in cell membrane.
Lecithin is one example of a phospholipid.
Living organisms have a number of carbon compounds in which heterocyclic rings can be found.
Some of these are nitrogenous bases -Adenine, guanine, cytosine, uracil and thymine.
When found attached to a sugar, they are called nucleosides.
If a phosphate group is also found esterified to the sugar they are called nucleotides.
Adenosine, guanosine, thymidine, uridine and cytidine are nucleosides.
Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid are nucleotides.
Only three types of macromolecules, i.e., proteins, nucleic acids and polysaccharides are found in living systems.
Lipids, because of their association with membranes separate in the macromolecular fraction. Biomacromolecules are polymers.
They are made of building blocks which are different.
Proteins are heteropolymers made of amino acids. Nucleic acids (RNA and DNA) are composed of nucleotides.
Biomacromolecules have a hierarchy of structures primary, secondary, tertiary and quaternary.
Nucleic acids serve as genetic material.
Polysaccharides are components of cell wall in plants, fungi and also of the exoskeleton of arthropods.
They also are storageforms of energy (e.g., starch and glycogen).
Proteins serve a variety of cellular functions.
Many of them are enzymes, some are antibodies, some are receptors, some are hormones and some others are structural proteins.
Collagen is the most abundant protein in animal world and Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO) is the most abundant protein in the whole of the biosphere.
Enzymes are proteins which catalyse biochemical reactions in the cells.
Ribozymes are nucleic acids with catalytic power.
Proteinaceous enzymes exhibit substrate specificity, require optimum temperature and pH for maximal activity.
They are denatured at high temperatures.
Enzymes lower activation energy of reactions and enhance the rate of the reactions greatly.
Nucleic acids carry hereditary information and are passed on from parental generation to progeny.
In some cases non-protein constituents called cofactors are bound to the enzyme to make the enzyme catalytically active.
In these instances, the protein portion of the enzymes is called the apoenzyme.
Three kinds of cofactors may be identified; prosthetic groups, coenzymes and metal ions.
Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are tightly bound to the apoenzyme.
For examples, in peroxidase and catalase, which catalyse the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a part of the active site of the enzyme.
Co-enzymes are also organic compounds but their association with apoenzyme is only transient, usually occurring during the course of catalysis.
NAD, NADP are co-enzymes and contain niacin vitamin.
A number of enzymes require metal ions for their activity which form coordination bonds with side chains at the active site e.g. zinc is a cofactor for the proteolytic enzyme carboxypeptidase.
Enzymes
Enzymes
Proteins make up almost all enzymes. Some nucleic acids have enzyme-like properties. Ribozymes are the name for these proteins. A line diagram can be used to represent an enzyme. An enzyme, like any other protein, has a primary structure, which is the amino acid sequence. Secondary and tertiary structures are present in enzymes, as they are in other proteins. When you look at a tertiary structure, you'll observe that the protein chain's backbone folds in on itself, the chain crisscrosses itself, and many crevices or pockets form. The 'active site' is one such pocket. An enzyme's active site is a crevice or pocket that the substrate fits into. As a result, enzymes catalyze processes at a rapid pace through their active site.
In many aspects, enzyme catalysts differ from inorganic catalysts, but one key distinction stands out. At high temperatures and pressures, inorganic catalysts perform efficiently, but enzymes are destroyed at high temperatures (say, above 40°C). Enzymes extracted from organisms that dwell in severe heat (e.g., hot vents and sulphur springs) are stable and keep their catalytic power even at extreme temperatures (up to 80°-90°C). The capacity of such enzymes extracted from thermophilic organisms to maintain their thermal stability is thus critical.
Chemical reactions
Chemical compounds go through two sorts of transformations. A physical change is merely a change in shape that does not involve the breaking of connections.This is a bodily function. A change in state of matter is another physical process, such as when ice melts into water or when water becomes vapour. These are physical processes as well. A chemical reaction, on the other hand, occurs when bonds are broken and new bonds are generated during transformation. For example
Ba (OH)2+ H2SO4 ® BaSO4 + 2H2O
is a chemical process that takes place in an inorganic state. The conversion of starch to glucose is also an organic chemical reaction. The amount of product created per unit time is referred to as the rate of a physical or chemical process. It can be written as:
rate=dP/ dT
If the direction is indicated, rate is also known as velocity. Temperature, among other things, affects the rates of physical and chemical processes. For every 10°C change in either direction, the rate doubles or declines by half, according to a common rule of thumb. Catalyzed reactions proceed at a much faster rate than uncatalyzed reactions. For every 10°C change in either direction, the rate of enzyme catalysed reactions would be substantially larger than the same but uncatalyzed reaction. Catalyzed reactions proceed at a much faster rate than uncatalyzed reactions. When enzyme-catalyzed reactions are detected, the rate is much higher than when the identical process is not catalysed. As an example,
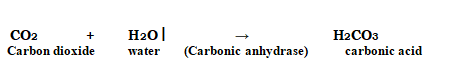
In the absence of any enzyme, this reaction is extremely sluggish, producing just around 200 molecules of H2CO3 per hour. The reaction speeds up substantially when carbonic anhydrase, an enzyme found in the cytoplasm, is used, with roughly 600,000 molecules generated every second. The enzyme has sped up the reaction rate by a factor of ten million. The power of enzymes is very amazing! Thousands of different enzymes exist, each catalysing a different chemical or metabolic reaction. A metabolic route is a multistep chemical reaction in which each step is catalysed by the same enzyme complex or separate enzymes. As an example,
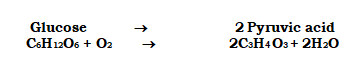
is a metabolic pathway in which glucose is converted to pyruvic acid via 10 enzyme-catalyzed chemical events. At this point, you should be aware that this metabolic pathway, when supplemented with one or two additional processes, produces a wide range of metabolic end products. Lactic acid is produced in skeletal muscle under anaerobic circumstances. Pyruvic acid is generated under typical aerobic circumstances. The similar route leads to the generation of ethanol in yeast during fermentation (alcohol). As a result, different products are possible depending on the circumstances.
How do Enzymes bring about such High Rates of Chemical Conversions?
The concept of an active site is already known. A reaction is referred to as a chemical or metabolic change. A'substrate' is the chemical that is transformed into a product. As a result, enzymes, which are three-dimensional proteins with a 'active site,' convert a substrate (S) into a product (P). This can be represented symbolically as
![]()
Within a specific cleft or pocket, the substrate 'S' must bind the enzyme at its'active site.' The substrate must diffuse into the 'active site.' As a result, the creation of a 'ES' complex is required. The letter E stands for enzyme. This intricate structure is a one-time occurrence. A new structure of the substrate called the transition state structure is created when the substrate is attached to the enzyme active site. The product is quickly removed from the active site after the intended bond breaking/making is completed. In other words, the substrate's structure is changed into the product's structure (s). This transformation must pass via a structure known as a transition state structure.Between the stable substrate and the result, there could be many additional 'altered structural states.' The notion that all other intermediate structural states are unstable is implicit in this assertion. Stability has to do with the molecule's or structure's energy status.
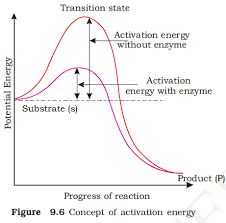
The potential energy content is shown by the y-axis. The x-axis depicts the structural transformation or states as they proceed through the 'transition stage.' There would be two things that you would notice. The difference in energy levels between S and P. When 'P' is less than 'S,' the reaction is exothermic. It is not necessary to provide energy (through heating) in order to manufacture the product. Regardless of whether the reaction is exothermic or spontaneous or endothermic or energy-demanding, the 'S' must pass through a significantly higher energy level or transition state. 'Activation energy' is the difference between the average energy content of 'S' and that of this transition state. Enzymes gradually break down this energy barrier, allowing the 'S' to become 'P' more easily.
Nature of Enzyme Action
In order to create a highly reactive enzyme-substrate complex (ES), each enzyme (E) has a substrate (S) binding site in its molecule. This complex has a brief half-life and dissociates into its product(s) P and the unmodified enzyme, with the enzyme-product complex forming in the middle (EP). Catalysis requires the creation of the ES complex.
E+S → ES → EP → E+P
An enzyme's catalytic cycle can be broken down into the following steps:
1. The substrate first binds to the enzyme's active site, fitting into the active site.
2. The enzyme's shape changes as a result of the substrate's binding, causing it to fit more tightly around the substrate.
3. The enzyme's active site, which is now in close proximity to the substrate, breaks the substrate's chemical bonds, forming a new enzyme-product complex.
4. The reaction products are released by the enzyme, and the free enzyme is ready to bind to another molecule of the substrate and repeat the catalytic cycle.
Factors Affecting Enzyme Activity
A change in the circumstances can impact the activity of an enzyme by altering the protein's tertiary structure. Temperature, pH, changes in substrate concentration, and the binding of certain molecules that affect its activity are examples of these.
Temperature and pH: Enzymes typically operate within a narrow temperature and pH range. The optimum temperature and pH for each enzyme are defined as the temperature and pH at which the enzyme is most active. Both below and above the ideal value, activity decreases. Because proteins are denatured by heat, low temperatures maintain the enzyme in a temporarily inactive state, but high temperatures eliminate enzymatic function.
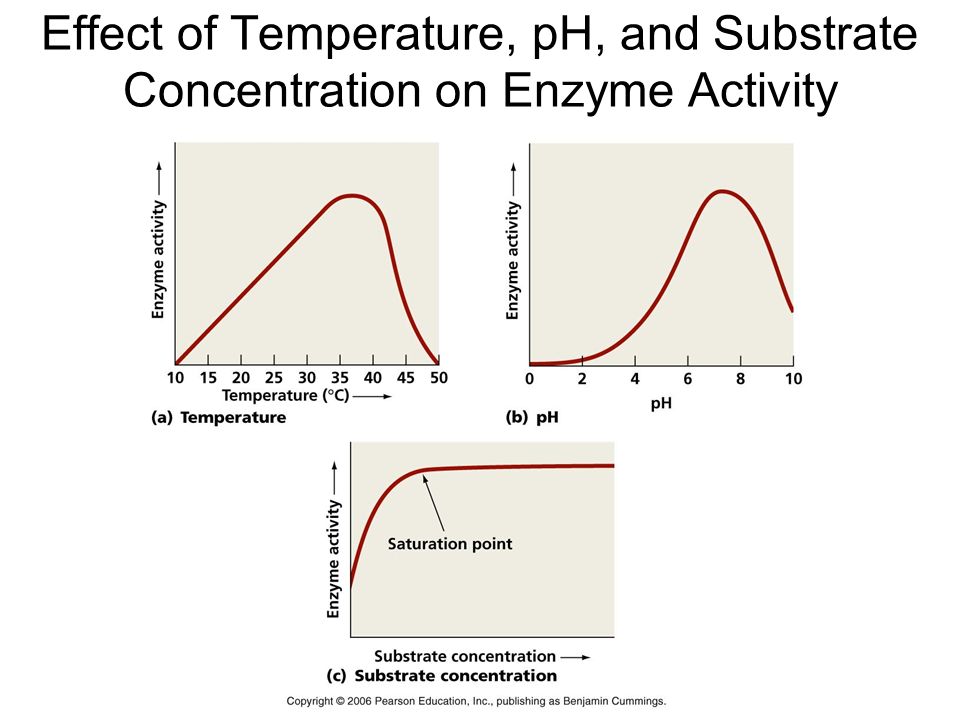
Substrate Concentration: At first, the enzymatic reaction's velocity increases as the substrate concentration rises. The reaction eventually achieves a maximum velocity (Vmax) that isn't exceeded by any further increase in substrate concentration. This is because there are fewer enzyme molecules than substrate molecules, and there are no free enzyme molecules to interact with the additional substrate molecules after these molecules have been saturated. An enzyme's activity is also affected by the presence of certain molecules that bind to it. The process of inhibition occurs when a chemical binds to an enzyme, and the chemical is referred to as an inhibitor.Competitive inhibitors are those that have a chemical structure that closely mimics that of the substrate and inhibits the enzyme's activity. Because of its structural similarity to the substrate, the inhibitor competes with it for the enzyme's substrate binding site. As a result, the substrate is unable to attach, and the enzyme's activity is reduced, as seen in the inhibition of succinic dehydrogenase by malonate, which is structurally similar to the substrate succinate. Competitive inhibitors like these are frequently employed to combat bacterial infections.
Classification and Nomenclature of Enzymes
There have been thousands of enzymes identified, isolated, and analyzed. The majority of these enzymes have been divided into groups depending on the kind of reactions that they catalyze. Enzymes are classified into six classes, each having four to thirteen subclasses, and given a four-digit number.
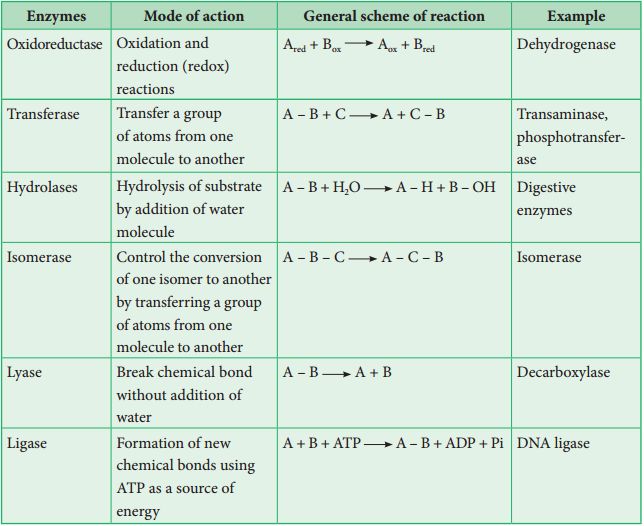
Co-factors
Enzymes are polypeptide chains made up of one or more polypeptide chains. However, in other situations, non-protein elements known as cofactors are coupled to the enzyme in order for it to be catalytically active. The apoenzyme refers to the protein part of the enzyme in these cases. Prosthetic groups, co-enzymes, and metal ions are all examples of cofactors. Prosthetic groups are chemical molecules that are strongly attached to the apoenzyme, distinguishing them from other cofactors. The prosthetic group haem is a component of the active site of enzymes like peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen.Co-enzymes are organic substances as well, although their relationship with the apoenzyme is very temporary, occurring most often during catalysis. Co-enzymes are also used as co-factors in a variety of enzyme-catalyzed processes. Many coenzymes contain vitamins as important chemical components; for example, the coenzymes nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin. Metal ions that create coordination bonds with side chains in the active site while also forming one or more coordination bonds with the substrate are required for the activity of a number of enzymes, for example, zinc is a cofactor for the proteolytic enzyme carboxypeptidase. When a co-factor is removed from an enzyme, its catalytic activity is lost, indicating that they play an important part in the enzyme's catalytic activity.
Phases of the Cell Cycle
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Phases of Cell Cycle
Cell division cycle or the cell cycle is a 4-stage process in a somatic cell during which two significant molecular processes occur – parent chromosome duplication (occurring in S phase) and equal detachment of the chromosome to the daughter cells (occurring during M phase)
In eukaryotic cells, the cell cycle phases are split into two significant phases – interphase and the mitotic phase. While in interphase, the cell significantly grows and replicates a DNA copy, in the mitotic phase or the M phase, the cell splits its DNA into two sets and hence the division of the cytoplasm to form two daughter cells.
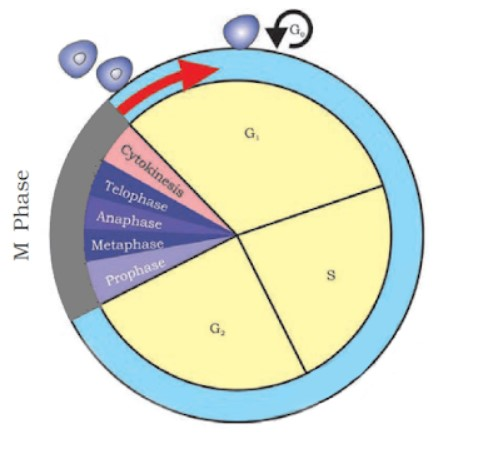
Interphase
Interphase is the time lapse between two successive M phases of cell division. The cell prepares for division, grows and DNA replication takes place. Interphase is further divided into three phases: G1, S, G2
G1 (Gap 1) Phase
This is the primary stage of the interphase, known as the G1 or first gap phase as diminutive changes are observed due to the hyperactivity of the cell at the biochemical degree
This phase is characterized by changes in the chromosomes from the condensed to the extended state in addition to a range of metabolic activities leading to the initiation of replication of DNA.
Characteristics of the chromatin fibres in this phase are – less coiled and slender, extended fully and ready for transcription. The process of transcription results in the production of RNAs and also a sequence of protein molecules vital for DNA replication to be initiated
G1 phase is lengthier than the other three phases and varies from cell to cell
This is a significant phase as cell grows and assembles building blocks of chromosomal DNA and the linked proteins. In addition, it also reserves adequate energy to accomplish chromosome replication
DNA synthesis in this phase is initiated at a distinct checkpoint. The cell progresses towards division once all the biochemical events at this particular point have concluded.
Synthesis (S) phase
It is an active DNA synthesis and histone synthesis phase of the interphase
Here the chromosomes replicate, enabled by linked proteins and DNA replication. Most of the histone protein synthesis occurs in this phase, though some of it occurs in G1 phase
Identical pair of DNA molecules are formed as the process of DNA replication is discontinuous and semi-conservative
Even after the chromosomes have doubled, the sister chromatids are securely attached to the centromeric region. The chromosome count of the cell remains the same
Centrosomes of animal cells at the centre of each animal cell is linked with centrioles positioned perpendicular to each other. The centrioles are functional in organizing the cell division process
During this phase, the centrosome is duplicated, producing the mitotic spindle, the apparatus which liaises chromosomal movement while mitosis is taking place
G2 (Gap 2) Phase
This phase is succeeded by the S phase. Here the chromosomes comprise two chromatids thus cell has double the quantity of DNA
Here, the cell restores its energy, producing proteins essential for chromosomes to manipulate
Few of the cell organelles are replicated. Cytoskeleton dismantles to render resources for mitosis
Additional growth of cell may be observed. Before the cell enters the first phase of mitosis, the concluding preparations of the mitotic phase must be done.
M (Mitotic) Phase
This phase is succeeded by the G2 phase. Here the cell divides into two daughter cells along with equal distribution of chromosomes between the daughter cells. Once the M phase steps into the G1 phase, the next cell cycle is initiated to be repeated. Some cells, however, do not enter into the G1 phase. These are referred to as G0 cells
It comprisesq the following sub-phases –
Prophase – in this stage, the nucleus disappears, spindle fibres are formed, DNA condenses into sister chromatids
Metaphase – the sister chromatids orient alongside the cell-equator by linking their centromeres to the spindle fibres
Anaphase – separation of sister chromatids at the centromere, being pulled towards the opposite poles of the cell by mitotic spindle
Telophase – At the opposite poles, the chromosomes arrive to unwind into fine DNA strands. Spindle fibres vanish. Nuclear membrane resurfaces
Cytokinesis – cell membrane splits, animal cells drift away. Plant cells form a cell plate which turns into a new cell wall
Cells arriving at the G0 phase, which is the inactive phase once they exit the cell cycle when they are not preparing actively to divide. Few of these cells tend to remain in this stage permanently.
Phases of the Cell Cycle
Phases of Cell Cycle
Human cells in culture provide an example of a normal eukaryotic cell cycle. Every 24 hours or so, these cells divide once. The length of the cell cycle can, however, differ from organism to organism and from cell type to cell type. For instance, yeast may complete the cell cycle in about 90 minutes.
Interphase and M Phase(Mitosis phase) are the two fundamental phases of the cell cycle.
Interphase
A cell spends the majority of its time in what is known as interphase, where it develops, duplicates its chromosomes, and gets ready to divide. The cell then exits interphase, goes through mitosis, and finishes dividing. The phases of interphase are G1 phase (cell growth), S phase (DNA synthesis), and G2 phase (cell growth). The mitotic phase, which consists of mitosis and cytokinesis and produces two daughter cells, begins after interphase. In the cell cycle, the interphase is a protracted resting phase during which , DNA is replicated, RNA is synthesised, and proteins are produced.
The transitional period between mitosis and the start of DNA replication is known as the G1 phase. The cell is metabolically active and continues to develop during the G1 phase but does not duplicate its DNA. The time when DNA synthesis or replication occurs is known as the "S" phase. The amount of DNA in each cell doubles throughout this period. DNA grows from 2C (the initial amount) to 4C (the final amount). However, the number of chromosomes does not grow; if the cell had diploid or 2n chromosomes at G1, the number of chromosomes continues to be 2n even after S phase.In animal cells, the centriole doubles in the cytoplasm and DNA replication starts in the nucleus during the S phase. Proteins are created during the G2 phase as cells continue to expand in preparation for mitosis.Thus interphase is vital in the cell cycle as it permits the cell to grow and develop into a mature cell before it is able to reproduce.
Heart cells are one type of cell that does not appear to divide in adult animals, and many other cells only sporadically divide when it is necessary to replenish cells lost to damage or cell death. These cells leave the G1 phase and enter the quiescent stage (G0), which is an inactive phase of the cell cycle. While still metabolically active, cells in this stage no longer divide unless specifically instructed to do so by the organism. Only the diploid somatic cells in animals undergo mitotic cell division. In contrast, both haploid and diploid cells in the plants can exhibit mitotic divisions.
M Phase
It is the most dramatic phase of the cell cycle and involves a significant reorganisation of almost all cell components. It is also known as equational division since both the parent and progeny cells have the same number of chromosomes. Although mitosis has been conveniently split into four stages of nuclear division, it is crucial to realise that cell division is a progressive process, and extremely obvious distinctions between different stages cannot be made. Two processes make up the M phase: cytokinesis (or cell division), in which a cell's cytoplasm splits in half to create two different daughter cells, and mitosis, in which the cell's chromosomes are divided equally between the two daughter cells.
Mitosis and it's significance
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
MITOSIS
It was first observed by Strasburger in plant cells and by Flemming in animal cells.
It may be defined as, "The exact replication of a parent cell into two identical daughter cells having the identical number, and kind of chromosomes, the identical DNA and identical hereditary instructions as found in the parental cell".
It mainly occurs in somatic cells of animals and in meristematic tissue cells of plants for multiplication of undifferentiated cells.
In mitosis the cell cycle involves series of changes which occur in a newly formed cell to become fully grown and to be ready for cell division.
It has two phases :
(1) Interphase (as described earlier)
(2) Mitotic phase or M-phase.
It consist of the following four sub-stages :
(a) Prophase: It is the first phase of mitosis which is longest of the M-phase.
Following changes occur during this stage:
(i) The chromatin fibres become shorter and thicker due to the process of coiling and folding and get condensed into distinct thread like chromosomes in late prophase.
(ii) Each chromosome appears double and consists of two coiled sister chromatids joined by a centromere. Their ends are not visible in early prophase. Therefore, the chromosomes appear like a ball of wool. It is also called spireme stage.
(iii) Cells at the end of prophase, when viewed under the microscope, do not show golgi complex, endoplasmic reticulum, nucleolus and the nuclear envelope.
(iv) The centriole now begins to move towards opposite poles.
(v) Fine radiating microtubules appear around each pair of centrioles to form the astral rays. A pair of centrioles and astral rays constitute a star like aster body.
(b) Metaphase
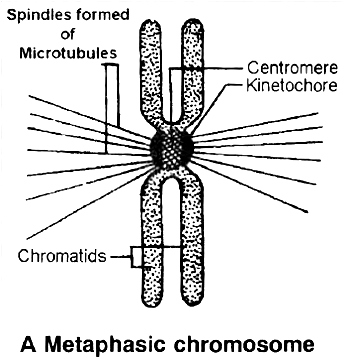
 (i) The complete disintegration of the nuclear envelope marks the start of the second phase of mitosis. By this stage condensation of chromosomes is completed. At this stage, metaphase chromosome appears to be made up of two sister chromatids, which are held together by the centromere.
(i) The complete disintegration of the nuclear envelope marks the start of the second phase of mitosis. By this stage condensation of chromosomes is completed. At this stage, metaphase chromosome appears to be made up of two sister chromatids, which are held together by the centromere.
(ii) Small disc shaped structures called kinetochores, serve as the sites of attachment of spindle fibres to the chromosomes that are moved into position at the centre of the cell.
Hence, the metaphase is characterised by all the chromosomes coming to lie at the equator and getting aligned along metaphase plate or equatorial plate through spindle fibres to both the poles.This chromosomal movement to equator is called as congression.
(iii) Spindle fibres which connect the pole to the chromosomes at the kinetochores are called chromosomal fibres (Tractile fibres) and those which extend without interruption from one pole to other are the continuous fibres.
(iv) The morphology of chromosomes can be easily studied at this stage as they are in a highly condensed stage and are distinctly visible. Chromosomes can be counted at this stage.
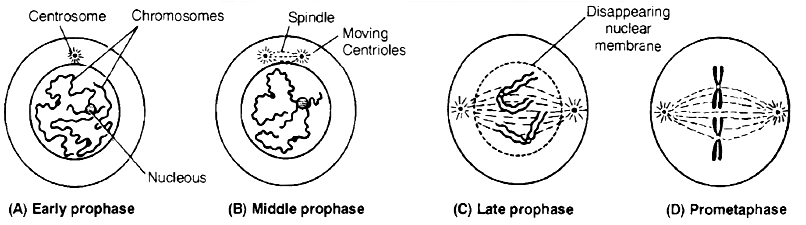
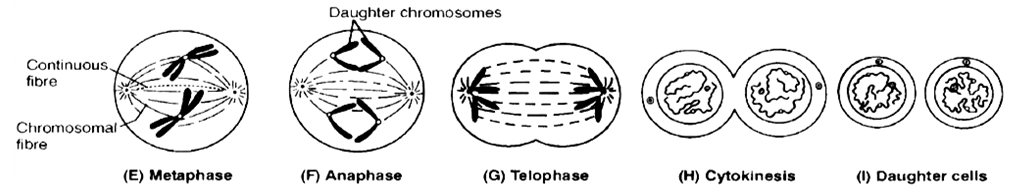
(c) Anaphase
(i) It is of shortest duration.
(ii) The centromere of each chromosome splits into two, resulting into daughter chromatids (chromosome of future nuclei).
(iii) These begin to migrate towards respective poles. The centromeres always lead the daughter chromosomes (towards the poles) and the arms trail behind.
(iv) As the daughter chromosomes move apart, fine microtubules in the form of interzonal fibres appear in between them.
(v) Expansion of interzonal fibres and dissolution of microtubules of chromosomal fibres is supposed to be the possible reason for anaphasic movement.
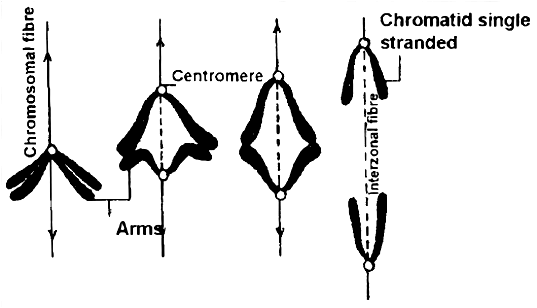
(vi) The daughter chromosomes now assume V, J and I shapes depending upon the position of centromere on them. Thus shape of chromosomes can be observed during this phase.
(vii) Finally, the polar migration of daughter chromosomes after reaching the opposite poles end, which mark the end of anaphase.
(d) Telophase :
(i) Daughter chromosomes undergo decondensation and uncoiling at each pole, so they lose their individuality.
(ii) The chromatin gets surrounded by discontinuous segments of nuclear membrane from elements of ER, which become fully developed at the end of telophase.
(iii) New nucleoli. ER and golgi complex reform.
(iv) The spindle fibres and astral rays gradually disintegrate and disappear. These become absorbed in the cytoplasm.
(v) Each daughter nucleus now enters into the interphase of cell cycle. The end of telophase marks the end of karyokinesis.
Cytokinesis : Karyokinesis results in formation of two nuclei inside a cell and now it is followed by division of cytoplasm (Cytokinesis), thus forming two cells (daughter cells).
(a) Cytokinesis occurs by two methods :
(i) Cell furrow method : This is characteristic of animal cells. Due to absence of rigid cell wall here, the more flexible plasma membrane forms the outer layer of cell. A furrow or invagination appears in plasma membrane at centre of equator, which deepens gradually and finally two daughter cells are separated.
(ii) Cell plate method : This is characteristic of plant cells. Here, vesicles provided by Golgi apparatus unite to form phragmoplasts, which join to form cell plate. Cell plate is first laid down in centre and then proceeds towards periphery (i.e., centrifugal plate formation). Cell wall materials are now laid down on both sides of cell plate, resulting in two daughter cells.
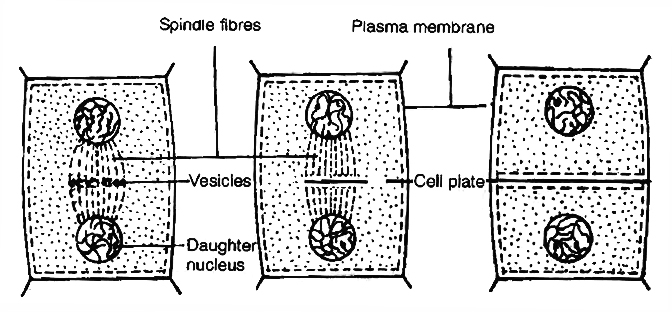
Cytokinesis by cell plate method in plant cells
Concept Builder
Mitotic Poisons
All those substances or chemicals which affect the cells during mitosis or prevent them from entering into normal mitotic divisions are called mitotic poisons. The various mitotic poisons are:
(i) The enzyme ribonuclease acts as poison at prophase. Azide and cyanide also inhibit prophase.
(ii) Mustard gas causes agglutination of chromosomes.
(iii) Chalones also inhibit mitosis. They are small peptides or glycoproteins in the extracellular fluid.
(iv) The alkaloid colchicine inhibits the formation of mitotic spindle (inhibits polymerisation of microtubules) and holds the cells in metaphase. The chromosomes and DNA undergo replication but remain within the same cell. The nucleus does not divide. This increases the number of chromosome sets per cell. This process leads to endopolyploidy or endomitosis in which nucleus contains multiple sets of chromosomes instead of the normal two sets as found in a diploid cell. Such cells are called polyploid cells.
(v) X-rays cause uncontrolled mitosis and induces breakage of chromosomes.
Abnormal Mitosis
(i) Intranuclear mitosis (Premitosis) : In Amoeba, Yeast, other fungi and a number of algae, the nuclear envelope does not degenerate during mitosis. Spindle is intranuclear.
(ii) Dinomitosis : Dinoflagellates possess condensed chromosomes even in non-dividing nuclei. Nuclear envelope does not degenerate. Division of chromosomes occurs with the help of special channels that develop in the nucleus.
(iii) Free Nuclear Division : Sometimes, repeated mitosis occur without subsequent cytokinesis. It produces multinucleated condition, e.g.:-Rhizopus, Vaucheria, Slime moulds, etc.
Significance of Mitosis
(i) It is an equational division since the number of chromosomes and the genetic constitution of the daughter cells remain the same as found in parent cells. Thus, mitosis helps in the survival of a species and continuation of its race. It provides a complete set of genetic information to each cell.
(ii) Mitosis occurs in somatic cells (n or 2n) and gonad cells for the multiplication of cell number. Thus, mitosis is related to the growth of an individual from zygote to adult stage. It also provides an opportunity for the growth and development of organs and body of organisms.
(iii) Mitosis produces new cells to replace the old worn out and injured cells. Thus, it helps in repairing of cells, healing of wounds and regeneration of body cells. Blood cells, intestinal cells and skin cells are regularly regenerated and replaced by mitosis.
(iv) It is a method of restoring nucleocytoplasmic ratio.
(v) Mitosis helps the organisms in both sexual and asexual reproduction.
Mitosis and it's significance
Mitosis and its significance
A cell prepares for cell division by replicating its chromosomes, segregating them, and creating two identical nuclei during the mitotic phase. The cell's contents are often evenly divided into two daughter cells with identical genomes after mitosis.Mitosis is divided into the following four stages:
1. Prophase:
Interphase's S and G2 phases are followed by prophase, the initial step of mitosis. The newly synthesised DNA molecules are not distinct but rather entangled in the S and G2 phases. The beginning of chromosomal material condensing characterises prophase. During the process of chromatin condensation, the chromosomal material is untangled. The centriole, which underwent duplication during interphase's S phase, now starts to migrate in the direction of the cell's opposing poles.Thus, the following distinctive occurrences can indicate the end of prophase:
- Compact mitotic chromosomes are created when chromosomal material condenses.Two chromatids are observed to be joined together at the centromere to form chromosomes.
- The beginning of the mitotic spindle's construction, the microtubules, and the proteinaceous elements of the cell cytoplasm aid in the process.
- The golgi complex, endoplasmic reticulum, nucleolus, and nuclear envelope are absent from cells near the end of prophase when they are observed under a microscope.
2. Metaphase:
The second phase of mitosis begins when the nuclear envelope completely disintegrates, and as a result, the chromosomes are dispersed throughout the cell's cytoplasm. The chromosome condensation process is now complete, and the chromosomes may be seen clearly under a microscope. Therefore, this is the period at which it is easiest to study the shape of chromosomes. The two sister chromatids that make up the metaphase chromosome at this point are joined by the centromere. Kinetochore refers to a little disc-shaped structure at the centromere surface. These structures act as the points of attachment for the spindle fibres, which are created by the spindle fibres, to the chromosomes that are placed at the cell's centre.Thus, all of the chromosomes align at the equator during the metaphase, with one chromatid of each chromosome attached by its kinetochore to spindle fibres from one pole and its sister chromatid connected by its kinetochore to spindle fibres from the opposite pole. The term "metaphase plate" refers to the chromosomes' alignment plane during metaphase. Metaphase's primary characteristics are:
- Spindle fibres adhere to chromosomal kinetochores.
- Chromosomes are transferred to the spindle equator, where they are positioned along the metaphase plate and along the spindle fibres to both poles.
3. Anaphase:
Each chromosome on the metaphase plate splits simultaneously at the start of anaphase, and the two daughter chromatids, which are now known as the chromosomes of the future daughter nuclei, start to move in opposite directions. The centromere of each chromosome is at the pole and, as a result, at the leading edge, with the arms of the chromosome trailing behind as they advance away from the equatorial plate. Events that define the anaphase stage are:
- Centromeres divide, and chromatids dissociate.
- Chromatids shift to the polar opposites
4. Telophase:
The chromosomes that have reached their respective poles decondense and lose their identity at the beginning of the last stage of mitosis, known as telophase. The individual chromosomes are no longer visible, and the two poles tend to accumulate a mass of chromatin material. The essential events at this stage include:
- Chromosomes cluster at opposing spindle poles, losing their identity as distinct elements.
- The nucleolus, Golgi complex, and ER remodel themselves
- The nuclear envelope forms around the chromosomal clusters.
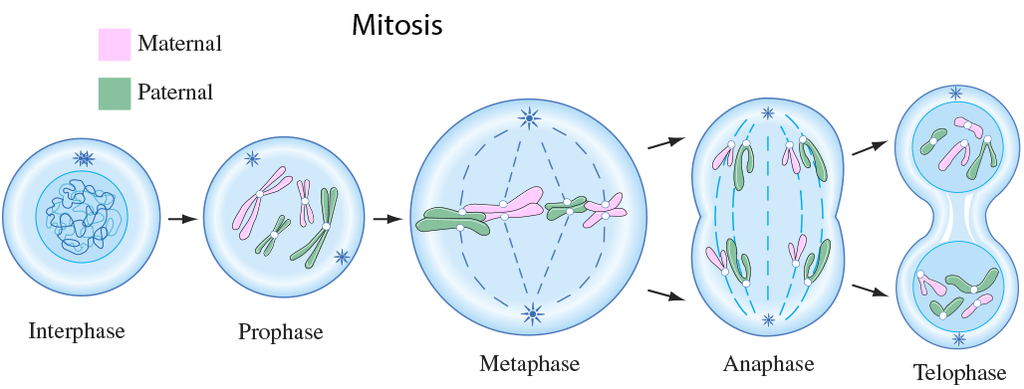
Cytokinesis:
In addition to segregating duplicated chromosomes into daughter nuclei (karyokinesis), mitosis also divides the cell into two daughter cells by a separate process known as cytokinesis, marking the completion of cell division. This occurs when a furrow forms in the plasma membrane of an animal cell. The cytoplasm of the cell is split in half by the furrow, which eventually merges in the centre. Plant cells, on the other hand, are surrounded by a cell wall that is rather inextensible; as a result, they go through cytokinesis using a different process. In plant cells, wall construction begins in the cell's middle and extends outward to meet the lateral walls that already exist.The basic precursor known as the cell-plate, which symbolises the middle lamella between the walls of two neighbouring cells, is formed before the new cell wall can be fully formed. Organelles like mitochondria and plastids are dispersed between the two daughter cells during cytoplasmic division. In some organisms, cytokinesis does not follow karyokinesis, which results in a multinucleate state and the development of syncytium.
Significance of Mitosis :
Only diploid cells often undergo mitosis, also known as equational division. However, haploid cells can also divide through mitosis in some lower plants and social insects. Understanding the importance of this division in an organism's life is crucial. Typically, mitosis produces daughter cells that are diploid and have the same genetic makeup. Mitosis is responsible for multicellular organisms' growth. The ratio of the nucleus to the cytoplasm is disturbed as a result of cell expansion. Therefore, cell division is required to re-establish the nucleo-cytoplasmic ratio. Cell repair is one of mitosis' most important functions.Blood cells, stomach lining cells, and the outermost layer of the epidermis all undergo continuous replacement. Plants grow continuously throughout their lives as a result of mitotic divisions in the meristematic tissues known as the apical and lateral cambium.
meiosis and it's significance
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
MEIOSIS
The term meiosis was first introduced by Farmer and Moore. It can be defined as, "The reductional division occurring only in diploid cells for the formation of haploid cells in which the number of chromosomes and the nuclear DNA content are reduced to half and there is recombination of hereditary material."
The key features of meiosis are as follows:
(a) It involves two sequential cycles of nuclear and cell division called meiosis I and meiosis II, but only a single cycle of DNA replication.
(b) Meiosis I can initiate only after the S phase (where parental chromosomes have replicated to produce identical sister chromatids)
(c) It involves pairing of homologous chromosomes and recombination between them.
(d) Four haploid cells are formed at the end of meiosis II.
(e) Meiotic events can be grouped under the following phases:
Meiosis-I Meiosis-II
Prophase-I Prophase-II
Metaphase-I Metaphase-II
Anaphase-I Anaphase-II
Telophase-I Telophase-II
Process of Meiosis
1. Meiosis I or Reductional or Heterotypic division : It starts after the interphase of the cell cycle where DNA duplication occurs in S phase. It results in the reduction of chromosome number to half.
Meiosis I consists of four stages, i.e., Prophase-I, Metaphase-I, Anaphase-I and Telophase-I.
(a) Prophase-I : It is very complex and of very long duration. It is divided into following substages on the basis of chromosome behaviour :
(i) Leptotene or leptonema :
The duplicated centrioles start moving apart. Aster formation occurs.
The chromatin fibres undergo progressive condensation, coiling, shortening and thickening to appear in the form of long, thin condensed filamentous chromosomes.
These possess darkly stained bead like structures called chromomeres along their entire length.
The chromosomes are replicated but the chromatids are not distinguishable due to the presence of nuclear protein between them.
The chromosomes form loops whose ends are attached to the nuclear membrane at attachment plate.
This specific arrangement of chromosomes is often called the "bouquet stage".
(ii) Zygotene or Zygonema or Synaptic stage:
The homologous chromosomes come to lie in pairs.
In each homologous pair of chromosomes, one chromosome comes from mother through ova and is called maternal chromosome and the other chromosome comes from father through sperm and is called paternal chromosome.
This pairing of homologous chromosomes is called synapsis and these paired chromosomes are called bivalents.
Homologous chromosomes in a pair are of the same length, carry the same genes in the same sequence.
Electron micrographs of this stage indicate that chromosome synapsis is accompanied by the formation of complex, tripartite, protienaceous structure called synaptonemal complex.
The number of bivalents in a cell is equal to the number of haploid chromosomes. The chromatids are still not visible.
(iii) Pachytene or Pachynema :
The bivalent chromosomes become more thickened, shortened and condensed.
Each chromosome of a bivalent consists of two chromatids and are called sister chromatids.
The two sister chromatids of one chromosome in a homologous pair with regard to the other chromosome of pair are called non-sister chromatids.
Four chromatids in a pair of homologous chromosomes constitute the tetrad.
This stage is characterised by the appearance of recombination nodules, the sites at which crossing over occurs between non-sister chromatids of the homologous chromosomes.
Crossing over is the exchange of genetic material between two homologous chromosomes and also an enzyme-mediated process and the enzyme involved is called recombinase.
The best theory to explain crossing over is Darlington's theory of breakage and union :
(a) The enzyme endonuclease will help in the development of breaks (nicking.)
(b) The formation of gaps in the nicks is done by exonuclease.
(c) The separation of chromatid segments in gaps is done by U-protein or unwindase.
(d) Re-annealing (rejoining) is done by R-protein or Re-annealing protein.
The newly constituted chromosomes, thus become different from the previous set of chromosomes.
This results in the formation of new characters (recombinants) and ultimately variations in the population. These variations form the basis of evolution.
(iv) Diplotene or Diplonema :
Longest phase of prophase-I. There is dissolution of synaptonemal complex, so the recombined homologous chromosomes will start separating i.e., desynapsis.
The separation of the homologous chromosomes is not completed.
They remain attached to one or more points where crossing over has occured.
These point of attachment are called as Chiasmata (X-shaped structures).
In oocytes of some vertebrates, the diplotene can last for months or years.
Suspended diplotene stage is called as Dictyotene stage.
At this stage the chromosomes decondense and are engaged in rapid synthesis of RNA. Terminalization of chiasmata starts.
(v) Diakinesis :
This stage is marked by complete terminalisation of chiasmata.
The nucleoli and the nucleolar membrane disintegrate and disappear.
The spindle fibres extend from one pole of paired centrioles to the other pole of paired centrioles.
Astral rays and asters become fully developed.
Centrioles are absent in plant cells and thus, no asters are formed.
This marks the end of prophase-I and transition to metaphase-I.
(b) Metaphase-I :
The bivalents arrange themselves on the equator of the bipolar spindle.
Since, there are two centromeres in each bivalent, the centromeres of all the bivalents produce a double equatorial or metaphasic plate.
Each plate will have half the number of diploid chromosomes.
The microtubule from the opposite poles of the spindle attach to the pair of homologous chromosomes.
(c) Anaphase-I :
One chromosome of each homologous pair moves to the opposite poles with recombined characters of both paternal and maternal chromosomes.
The movement of chromosomes occurs along the path of their tractile fibres (chromosome fibres).
Each chromosome consists of two chromatid threads joined by a centromere.
There is no division of centromere.
At the end of anaphase-I half of the chromosomes reach one pole and other half reach on to the other pole.
There occurs true reduction in the number of chromosomes at this stage.
(d) Telophase-I :
The haploid number of chromosomes which has reached at each pole, undergo elongation, uncoiling and decondensation and changes into chromatin network.
Although in many cases the chromosomes do undergo some dispersion, they do not reach the extremely extended state of the interphase nucleus.
The nuclear envelope develops from the elements of ER around the chromatin fibres. New nucleolus is formed.
The astral rays and spindle fibres disintegrate and disappear.
The two daughter nuclei, each containing haploid number of chromosomes are formed.
The end of telophase-I marks the end of karyokinesis-I also.
Karyokinesis-I may be followed by cytokinesis-I.
By a cleavage furrow in an animal cell and a cell plate in a plant cell, the diploid parental cell is divided into two haploid daughter cells each having half the number of chromosomes, but double content of DNA.
Interkinesis or intrameiotic interphase :
Each daughter cell formed from meiosis-I sometimes undergoes interkinesis, where there is no replication phase of chromosomes and no duplication of genes.
2. Meiosis II or Homotypic Division : It resembles mitotic division.
Meiosis-II is necessary because each daughter cell formed from meiosis-I contains chromosomes, each having two chromatids, each chromatid with 2C amount of DNA. This reduction in DNA from 2C to 1 C DNA occurs only in Meiosis-II.
It consists of following four phases :
(a) Prophase-II: One pair each of the centrioles migrate to the opposite poles. The nuclear membrane and the nucleolus disintegrate and disappear. The chromatin fibres undergo compaction to appear in the form of distinct chromosomes. Each chromosome consists of two chromatid threads, joined by a centromere.
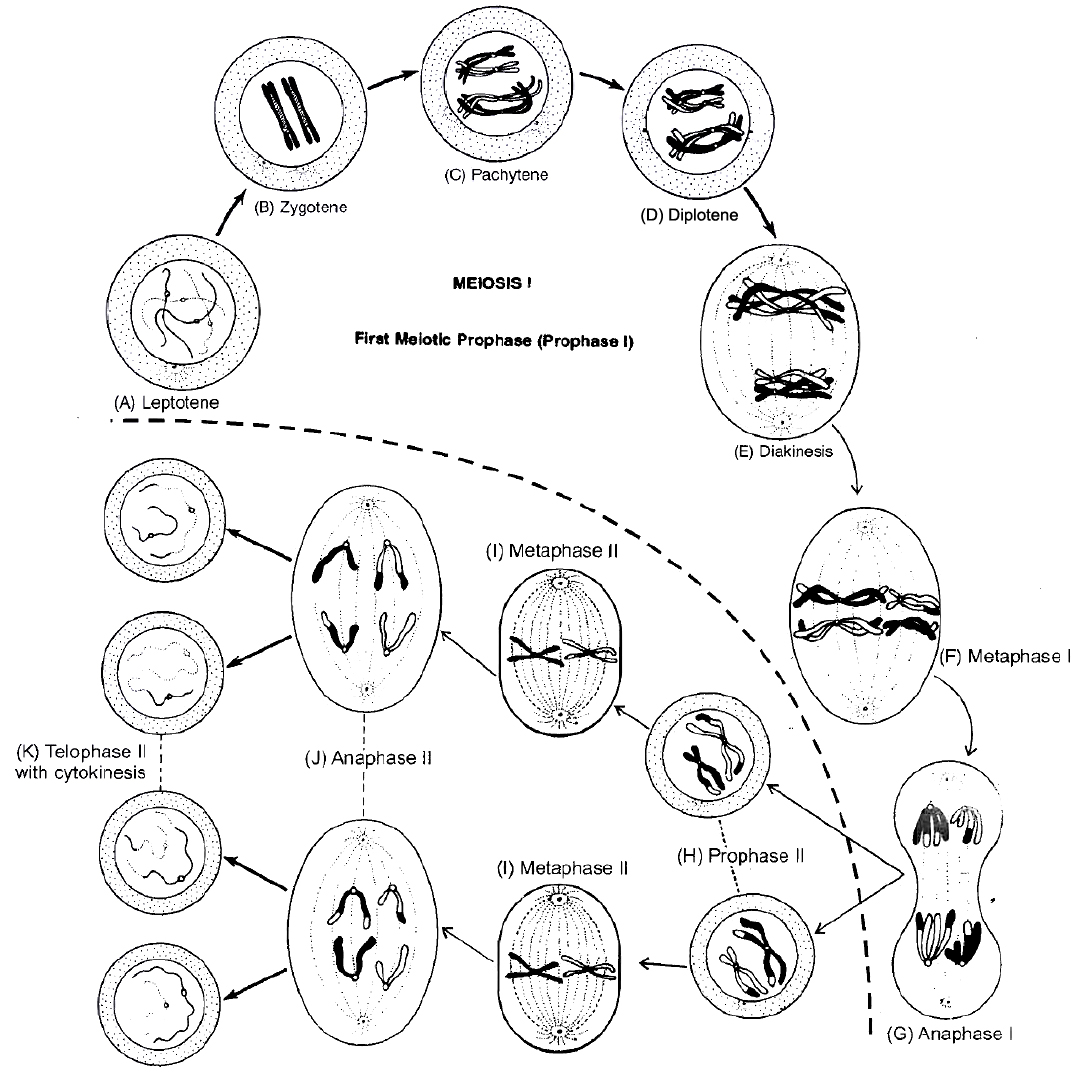
Different Stage of Meiosis
(b) Metaphase II : Chromosomes come to lie on the equator of the cell and thus, form a Single equatorial or metaphasic plate. The spindle fibres become attached to both kinetochores of the centromere of each chromosome.
(c) Anaphase-II: Begins with simultaneous splitting of the centromere of each chromosome that allows them to move towards opposite poles of the cell.
(d) Telophase-II: The daughter chromosomes on the opposite poles become decondensed to form chromatin fibres. The nuclear envelope develops from ER. New nucleolus is reorganised. The spindle fibres and astral rays disintegrate and disappear. It marks the end of the telophase-II and karyokinesis-II . It is followed by cytokinesis (similar in mitosis) which divides each cell into two daughter cells, resulting in formation of tetrad of cells.
Concept Builder
(i) Meiosis is commonly studied using onion buds.
(ii) Meiosis was first demonstrated by Van Benden and described by Winiwarter.
(iii) Gametic meiosis is also called as terminal meiosis.
(iv) Zygotic meiosis is also called as initial meiosis.
(v) Sporogenic meiosis is also called as intermediate meiosis.
(vi) Cytokinesis: Cytokinesis can be of two types, successive and simultaneous. In successive type, cytokinesis occurs after every nuclear division. The four cells formed by successive cytokinesis can be arranged either in a linear or isobilateral tetrad.
In the simultaneous type, cytokinesis takes place only at the end of both the divisions. The nuclei are generally arranged in the form of a tetrahedron.
Significance of Meiosis
1. Meiosis forms gametes that are essential for sexual reproduction.
2. Meiosis maintains the fixed number of chromosomes generation after generation in sexually reproducing organisms. It is essential since the chromosome number becomes double after fertilization.
3. Meiosis is the main cause of production of variations, which are very important for evolutionary process.
Some Major Differences between Mitosis and Meiosis
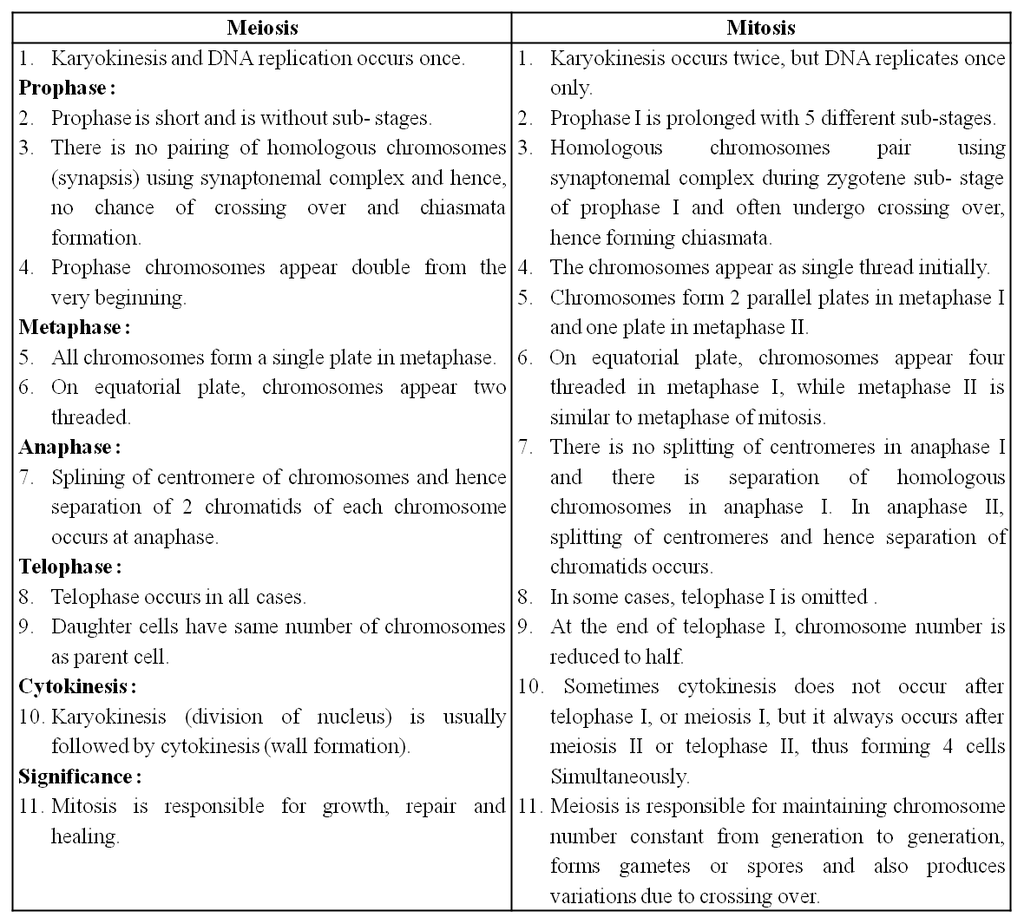
AMITOSIS OR DIRECT CELL DIVISION
It is the method of asexual reproduction, which occurs, in acellular organisms like bacteria, protozoans, diseased cells, old cells, mammalian cartilage cells and in foetal membranes.
it was first discovered by Remak. It is also called direct cell division.
During amitosis, the nucleus of the cell elongates.
Then, a constriction appears in the nucleus which gradually deepens and divides the nucleus into two daughter nuclei.
Finally, a constriction appears in the cytoplasm which divides the cytoplasm and the nuclei into two daughter cells, each with a nucleus.
In this division, no spindle formation and no distinct chromosome formation occurs. Nuclear envelope remains intact.
The daughter cells are approximately the two equal halves of a parental cell.
Concept Builder
(i) Meiosis is commonly studied using onion buds.
(ii) Meiosis was first demonstrated by Van Benden and described by Winiwarter.
(iii) Gametic meiosis is also called as terminal meiosis.
(iv) Zygotic meiosis is also called as Initial meiosis.
(v) Sporogenic meiosis is also called as Intermediate meiosis.
(vi) If we have to calculate the number of mitotic divisions for the formation of n number of cell, it will be n-1 i.e. for getting 100 cells 99 mitotic divisions are required.
(vii) Number of generations of mitosis required can be calculated (Like mitosis generations required for producing 128 cells are 7) using 2n, n is number of generation.
(viii) In animal cell, mitosis is called as Amphlastral (Spindle is associated with 2 asters).
(ix) In plant cells, the mitosis is called as Anastral (no aster, no centriole).
(x) If mitosis is extranuelear, it is Eumitosls.
(xi) If mitosis is intranuclear, it is called as Premitosis. If centrioles are present then it is called as centric.
(xii) Cytokinesis: Cytokinesis can be of two types, successive and simultaneous. In successive type, cytokinesis occurs after every nuclear division. The four cells formed by successive cytokinesis can be arranged either in a linear or isobilateral tetrad.
In the simultaneous type, cytokinesis takes place only at the end of both the divisions. The nuclei are generally arranged in the form of a tetrahedron.
Formulae Chart :
1. Number of mitotic division for the formation of n number of cells.
Example : For getting 100 cells 99 mitotic division are required.
![]()
2. Number of generations (n) of mitosis for producing 'x' cells.
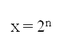
3. Number of meiosis for the formation of 'n' seeds/grains/fruits.
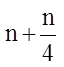
SUMMARY
1. According to the cell theory, cells arise from pre-existing cells. The process by which this occurs is called cell division.
2. Any sexually reproducing organism starts its life cycle from a single-celled zygote.
3. Cell division does not stop with the formation of the mature organism but continues throughout its life cycle.
4. The stages through which a cell passes from one division to the next is called the cell cycle.
5. Cell cycle is divided into two phases called (i) Interphase -a period of preparation for cell division, and (ii) Mitosis (M phase) -the actual period of cell division.
6. Interphase is further subdivided into G1, Sand G2. G1-phase is the period when the cell grows and carries out normal metabolism. Most of the organelle duplication also occurs during this phase.
7. S-phase marks the phase of DNA replication and chromosome duplication.
8. G2-phase is the period of cytoplasmic growth.
9. Mitosis is also divided into four stages namely prophase, metaphase, anaphase and telophase.
10. Chromosome condensation occurs during prophase.
11. Simultaneously, the centrioles move to the opposite poles.
12. The nuclear envelope and the nucleolus disappear and the spindle fibres start appearing.
13. Metaphase is marked by the alignment of chromosomes at the equatorial plate.
14. During anaphase, the centromeres divide and the chromatids start moving towards the two opposite poles.
15. Once the chromatids reach the two poles, the chromosomal elongation starts, nucleolus and the nuclear membrane reappear. This stage is called the telophase.
16. Nuclear division is then followed by the cytoplasmic division and is called cytokinesis.
17. Mitosis thus, is the equational division in which the chromosome number of the parent is conserved in the daughter cell.
18. In contrast to mitosis, meiosis occurs in the diploid cells, which are destined to form gametes. It is called the reduction division since it reduces the chromosome number by half while making the gametes.
19. In sexual reproduction when the two gametes fuse the chromosome number is restored to the value in the parent.
20. Meiosis is divided into two phases -meiosis-I and meiosis-II. In the first meiotic division the homologous chromosomes pair to form bivalents, and undergo crossing over.
21. Meiosis I has a long prophase, which is divided further into five phases. These are leptotene, zygotene, pachytene, diplotene and diakinesis.
22. During metaphase-I , the bivalents arrange on the equatorial plate.
23. In anaphase-I, homologous chromosomes move to the opposite poles with both their chromatids. Each pole receives half the chromosome number of the parent cell.
24. In telophase-I, the nuclear membrane and nucleolus reappear.
25. Meiosis-II is similar to mitosis.
26. During anaphase-II, the sister chromatids separate. Thus at the end of meiosis, four haploid cells are formed.
meiosis and it's significance
Meiosis and its significance
Meiosis is a type of cell division that results in the production of four gamete cells and a 50% reduction in the number of chromosomes in the parent cell. To develop egg and sperm cells for sexual reproduction, this process is necessary. There are four haploid daughter cells formed during meiosis (containing half as many chromosomes as the parent cell).Following are the main characteristics of meiosis:
- Meiosis requires just one cycle of DNA replication but two successive cycles of nuclear and cell division called meiosis I and meiosis II.
- After the paternal chromosomes have duplicated to form identical sister chromatids at the S phase, meiosis I begins.
- Meiosis involves the pairing and recombination of homologous chromosomes.
- At the end of meiosis II, four haploid cells are produced.
Events of Meiosis are classified as:
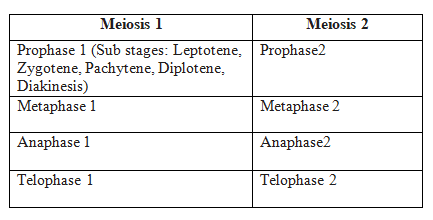
Meiosis 1
Prophase I: When compared to the prophase of mitosis, the prophase of the first meiotic division is often longer and more complex. Based on chromosomal behaviour, it has been further split into the following five phases: Leptotene, Zygotene, Pachytene, Diplotene, and Diakinesis.
Leptotene: The chromosomes begin to condense at this stage and are connected to the nuclear membrane via their telomeres.
Zygotene: A synaptonemal complex forms between homologous chromosomes at the start of synapsis.
Pachytene - During this stage, genetic material is transferred between chromatids that are not sisters. This is called crossing over.
Diplotene - At this stage, homologous pairs are still connected at the chiasmata after synapsis has ended and the synaptonemal complex has vanished.
Diakinesis: Before metaphase 1, the nuclear membrane finally breaks down and the chromosomes are entirely condensed.
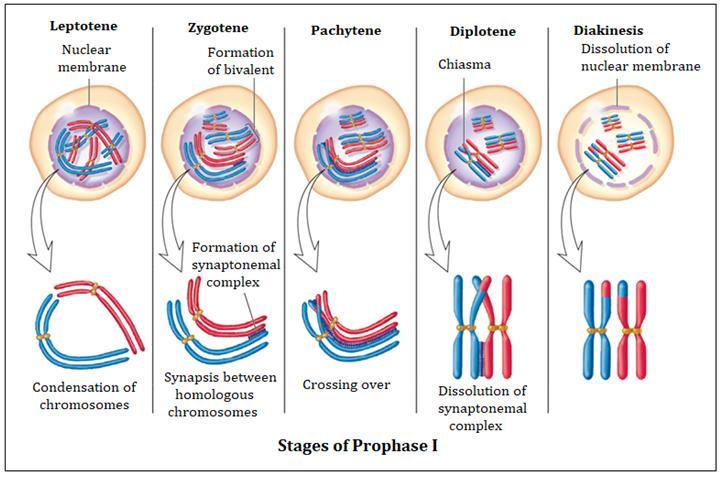
Metaphase I: The bivalent chromosomes line up on the equatorial plate during metaphase I. The spindle's opposing poles' microtubules connect to the pair of homologous chromosomes.
Anaphase I: Sister chromatids are still connected at their centromeres during anaphase I, but homologous chromosomes split.
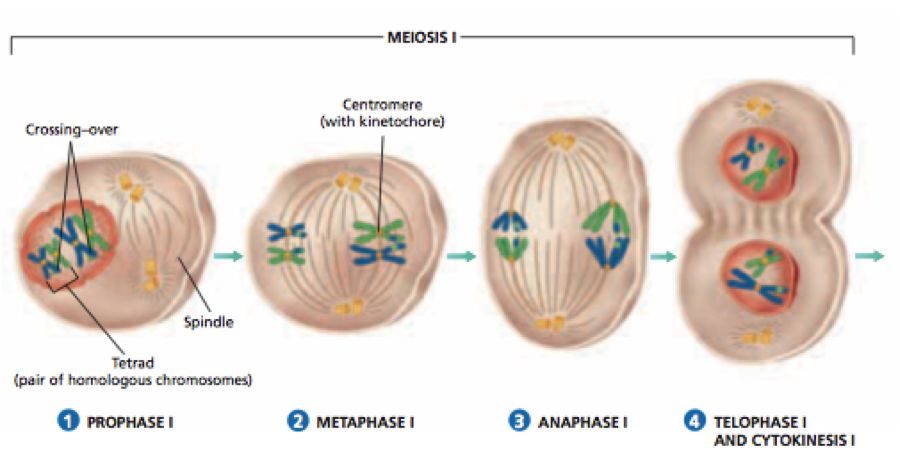
Telophase I: Cytokinesis occurs after the nuclear membrane and nucleolus re-emerge, and this is known as the "diad of cells." Even though the chromosomes do experience some dispersion in many instances, they rarely reach the interphase nucleus's very stretched state. Interkinesis, which occurs between the two meiotic divisions, is typically a transient stage. Prophase II, which is substantially less complex than prophase I, comes after interkinesis.
Meiosis 2:
Prophase II: Following cytokinesis, meiosis II begins right away, typically before the chromosomes have fully expanded. Meiosis II resembles a typical mitosis in contrast to meiosis I. By the end of prophase II, the nuclear membrane is gone. Chromosomes once more become condensed.
Metaphase II: Chromosomes align at the equator during metaphase II, and sister chromatids' kinetochores get attachments of microtubules from the spindle's opposing poles.
AnaphaseII: Beginning with the simultaneous division of each chromosome's centromere (which had been binding the sister chromatids together), anaphase II allows the chromosomes to migrate toward their respective poles of the cell.
Telophase II: Telophase II marks the completion of meiosis, during which the two chromosomal groups are once more encased in a nuclear membrane. Cytokinesis then takes place, culminating in the production of a tetrad of cells, or four haploid daughter cells.
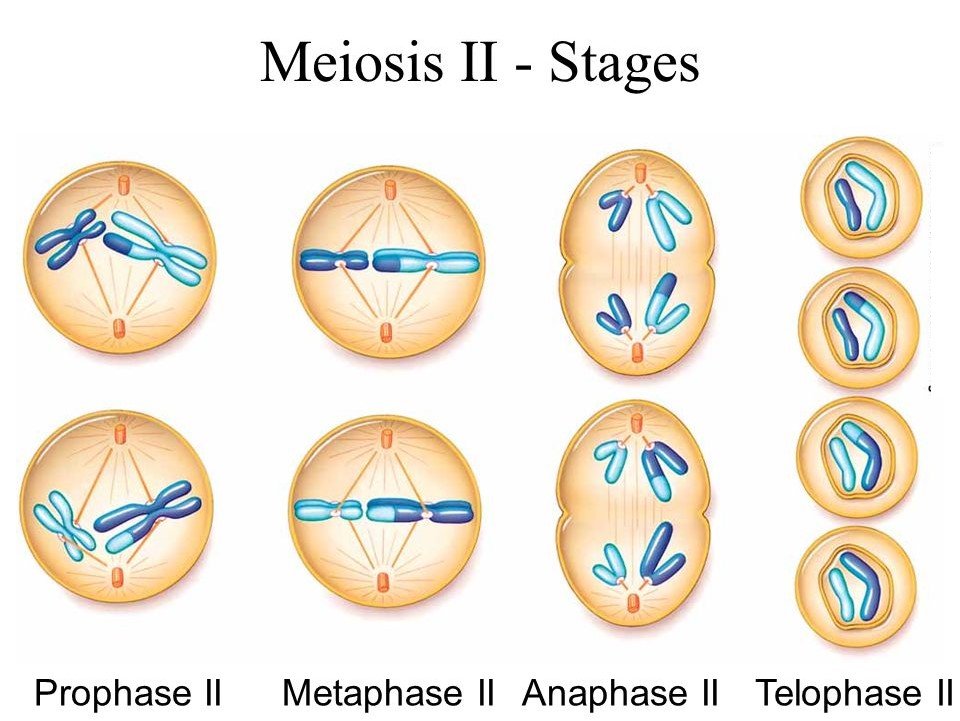
Significance of Meiosis:
Even though the process itself paradoxically reduces the number of chromosomes by half, meiosis is the mechanism that allows sexually reproducing animals to maintain the particular chromosomal number of each species throughout generations. From one generation to the next, it also makes the population of organisms more genetically variable. For the process of evolution, variations are crucial.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
