- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
MUSCLE CONTRACTION
When a nerve impulse (nerve action potential) reaches the synaptic end bulbs, it triggers exocytosis of synaptic vesicles.
In this process, the synaptic vesicles fuse with the plasma membrane and liberate ACh, which diffuses into the synaptic cleft between the motor neuron and the motor end plate.
When ACh binds to its receptor, a channel that passes small cations, most importantly Na+ opens.
The inrush of Na+ changes the resting membrane potential, which triggers a muscle action potential that travels along the muscle cell plasma membrane and initiates the events leading to muscle contraction.
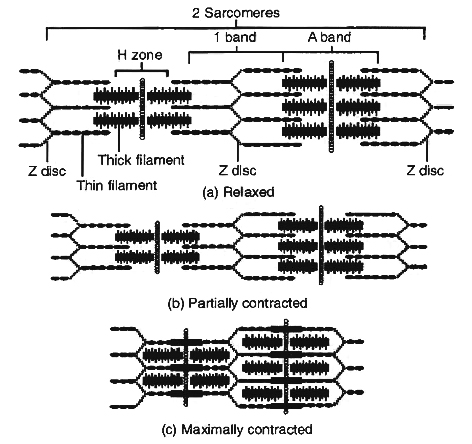
Hanson and Huxley proposed that skeletal muscle shortens during contraction because thin filaments slide over thick filament.
Their model is known as the sliding filament mechanism of muscle contraction.
Sliding Filament Mechanism
During muscle contraction, myosin heads pull on the thin filaments, causing them to slide inward towards the H zone at the centre of the sarcomere.
The myosin cross bridges may even pull the thin filaments of each sarcomere so far inward that their ends overlap in the centre of the sarcomere.
As the thin filaments slide inward, the Z discs come towards each other, and the sarcomere shortens, but the lengths of the thick and thin filaments do not change.
The sliding of the filaments and shortening of the sarcomeres cause shortening of the whole muscle fibre and ultimately the entire muscle.
Role of Calcium and Regulator Proteins
An increase in Ca2+ concentration in the sarcoplasm starts filament sliding, while a decrease turns off the sliding process.
When a muscle fibre is relaxed (not contracting), the concentration of Ca2+ in its sarcoplasm is low.
This is because the sarcoplasmic reticulum (SR) membrane contains Ca2+ active transport pumps that move Ca2+ from the sarcoplasm into the SR.
Ca2+ is stored or sequestered inside the SR.
As a muscle action potential travels along the sarcolemma and into the transverse tubule system, however, Ca2+ release channels open in the SR membrane.
As a result, Ca2+ floods into the sarcoplasm around the thick and thin filaments.
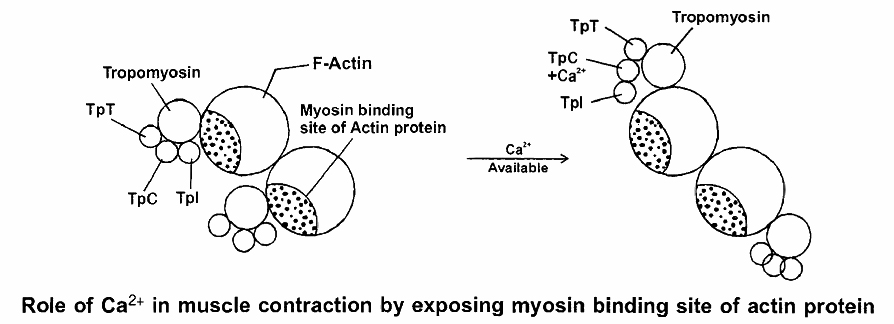
The Ca2+ released from the sarcoplasmic reticulum combine with troponin, causing it to change shape.
This shape change moves the troponin -tropomyosin complex away from the myosin-binding sites on actin.
Troponin has 3 units (tri units structure)
1. TpT - tropomyosin binding troponin
2. TpC - calcium binding protein
3. Tpl - Inhibitor i.e., blocks myosin binding site of actin proteins
The Power Stroke and the Role of ATP
As we have seen, muscle contraction requires Ca2+ ions and energy in the form of ATP. The sequence of events during sliding of the filaments are
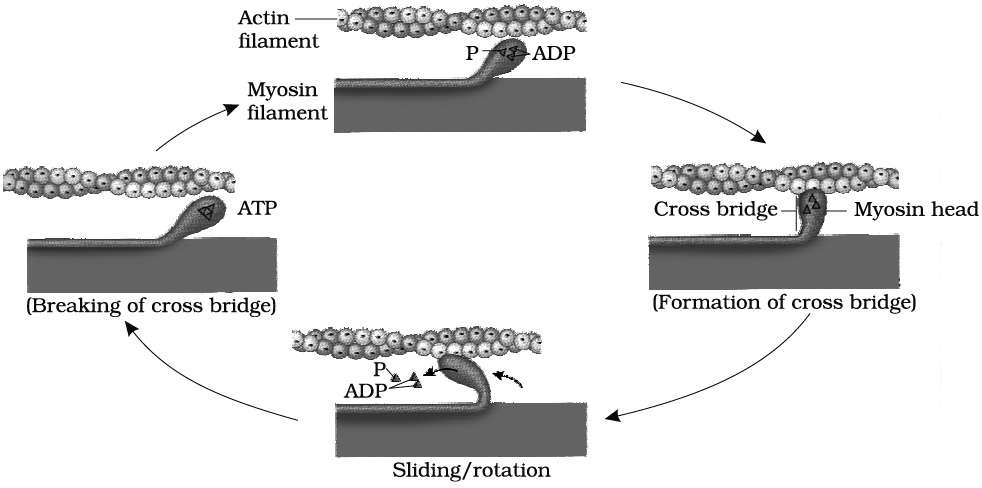
(i) While the muscle is relaxed, ATP attaches to ATP-binding sites on the myosin cross bridges (heads). A portion of each myosin head acts as an ATPase, an enzyme that splits the ATP into ADP + P (phosphate group) through a hydrolysis reaction. This reaction transfers energy from ATP to the myosin head, even before contraction begins. The myosin cross bridges are thus in an activated (energized) state.
(ii) When the sarcoplasmic reticulum releases Ca2+, its level rises in the sarcoplasm. Rise in Ca+2 binds with troponin and change its configuration that moves away tropomyosin from its blocking position.
(iii) The activated myosin heads spontaneously bind to the myosin-binding sites on actin.
(iv) The shape change that occurs as myosin heads bind to actin produces the power stroke of contraction. During the power stroke, the myosin heads swivel toward the centre of the sarcomere, like the oars of a boat during rowing. This action draws the thin filaments past the thick filaments toward the H zone. As the myosin heads swivel, they release ADP.
(v) Once the power stroke is complete, ATP again combines with the ATP-binding sites on the myosin heads. As ATP binds, the myosin head detaches from actin.
(vi) Again, the myosin ATPase splits ATP, transferring its energy to the myosin ATPase splits head, which returns to its original upright position .
(vii) The myosin head is then ready to combine with another myosin-binding site further along the thin filament.
The steps (iii) through (vii) repeats over and over as long as ATP is available and the Ca2+ level near the thin filament is high.
The myosin heads keep rotating back and forth with each power stroke, pulling the thin filaments toward the H zone.
At anyone instant, about half of the myosin heads are bound to actin and are swiveling.
The other half are detached and preparing to swivel again.
This continual movement of myosin heads applies the force that draws the Z discs toward each other, and the sarcomere shortens.
The myofibrils thus contract and the whole muscle fibre shortens.
During a maximal muscle contraction, the distance between Z discs can decrease to half the resting length.
H-line and M-line disappear, I-band almost disappears, A-band remains constant, but the power stroke does not always result in shortening of the muscle fibres and the whole muscle.
Contraction without shortening is called an isometric contraction, for example, in trying to lift a very heavy object.
The myosin heads (cross bridges) swivel and generate force, but the thin filaments do not slide inward.
Relaxation: Two changes permit a muscle fibre to relax after it has contracted.
First, acetylcholine is rapidly broken down by an enzyme called acetylcholinesterase (AChE).
When action potentials cease in the motor neuron, release of ACh stops, and AChE rapidly breaks down the ACh already present in the synaptic cleft.
This ends the generation of muscle action potentials, and the Ca2+ release channels in the sarcoplasmic reticulum membrane close.
Second, Ca2+ active transport pumps rapidly remove Ca2+ from the sarcoplasm into the sarcoplasmic reticulum, where molecules of a calcium-binding protein, appropriately called calsequestrin, bind to the Ca2+.
With this, the tropomyosin-troponin complex move back over the myosin binding site of actin which prevents further binding of myosin head to actin and the thin filaments slide back to their normal relaxed position.
All or None Principle
A minimal strength of a stimulus required to cause the contraction of a muscle fibre brings about maximum contraction, and no further increase in contraction would occur by increasing the strength of the stimulus.
Single Muscle Twitch
A Single, quick isolated contraction of a muscle fibre to a single stimulus of threshold value is called single muscle twitch in the laboratory experiments.
Energy Source of Muscle Contraction
Energy for muscle contraction is provided by hydrolysis of ATP by myosin ATP-ase enzyme.
This hydrolysis produces ADP, inorganic phosphate and energy (used in muscle contraction).
Phosphocreatine donates its high energy and phosphate to ADP, producing ATP.
Phosphocreatine serves as an energy source for a few seconds for metabolic processes in the muscle cells to begin to produce greater quantities of ATP.
Phosphocreatine is again formed in relaxing muscle by using ATP produced by carbohydrate oxidation.
ATP + H2O ![]() ADP + Pi + Energy
ADP + Pi + Energy
(Inorganic phosphate)
Phosphocreatine + ADP ![]() ATP + Creatine
ATP + Creatine
At the end of muscle contraction, the conversion of ADP into ATP takes place.
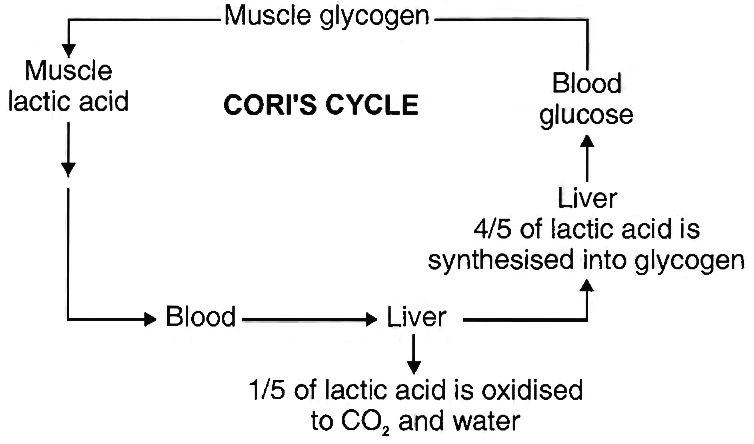
The muscle is rich in glycogen which is broken down into lactic acid through a series of reactions (glycolysis) and liberates energy.
Some of this energy is used for the reformation of phosphocreatine and also for the conversion of 4/5th of lactic acid back into glycogen.
The 1/5th of lactic acid is oxidised to water and carbon dioxide.
These reactions taking place in the muscle and liver, are proposed by Cori and Cori, hence known as Cori's cycle.
Rigor Mortis
Extreme rigidity of body after death is called rigor mortis. It is due to complete depletion of ATP and phosphocreatine.
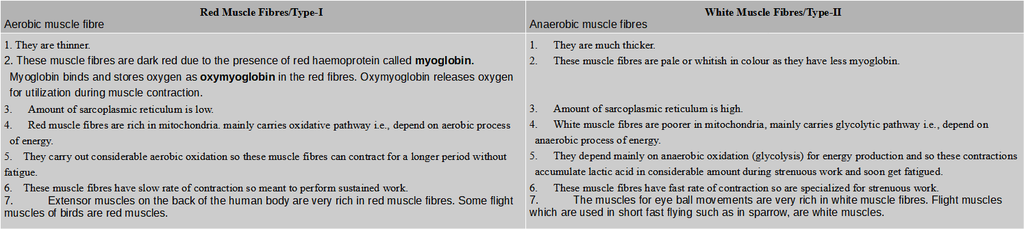
Red and White Muscle Fibres
Birds and mammals have two kinds of striated muscle fibres in their skeletal muscles; red or slow muscle fibres and white or fast muscle fibres.
Differences between red muscle fibres and white muscle fibres
Contraction in Smooth Muscles:
In comparison with contraction in a skeletal muscle fibre, contraction in a smooth muscle fibre starts more slowly and lasts much longer Troponin is absent in smooth muscle so they have regulator protein called Calmodulin that binds to Ca2+ in the cytosol.
Using ATP, myosin head part can bind to actin & contraction can occur.
Most smooth muscle fibres contract or relax in response to action potentials from the autonomic nervous system.
Contraction in Cardiac Muscles:
Cardiac muscle fibres have the same arrangement of actin & myosin and the same bands, zones and Z discs as skeletal muscle fibres.
Gap junctions allow muscle action potential to spread from one muscle fibre to another.
As a consequence, when a single muscle fibre is stimulated, all the other fibres in the network become stimulated as well.
Thus, each network contracts as a functional unit.
Cardiac muscle tissue has a long refractory period and can use lactic acid produced by skeletal muscle fibres to make ATP.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
