methods to study the mineral requirements of plants
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Methods to study the mineral requirements of plants
Soils normally contain sufficient quantities of essential minerals.
However, three important elements need to be replenished in crop fields as they are depleted by repeated cultivation.
These fertiliser elements called critical elements are nitrogen, phosphorus and potassium (NPK).
The common sources of these elements used in India are: nitrate of sodium, ammonium sulphate, ammonium nitrate, ammonium chloride, urea, etc.
The NPK fetilisers comprising bags of fertilisers are labelled 17-18-19 or 15-15-15 or other combinations.
These numbers refer to the percentage by weight of nitrogen, phosphorus and water soluble potassium.
To determine the elements essential for plant growth and deficiency symptoms of an essential element, well defined nutrient medium has to be used.
Seeds are grown in highly washed pure sand in a glass or glazed procelain or plastic container and supplied with a carefully made up nutrient solution.
Arnon and Hoagland's Medium prescribed a medium containing micronutrients.
Iron was earlier supplied as ferrous sulphate, but it often precipitated out.
This problem has now been solved by dissolving the ferrous sulphate along with a chelating agent Na-EDTA (disodium salt of ethylene diaminetetra acetic acid.)
Solution Culture
It is performed in glass jars or polythene bottles.
The container is covered with black paper after pouring solution into them.
Black paper has two functions -(a) Prevention of growth of algae (b) Prevention of reaction of roots with light.
Seeds are allowed to germinate over split cork.
Cotyledons are removed after seedling formation.
The plant is properly supported with the help of split cork.
Solution is aerated at regular intervals and is changed after 2-3 days.
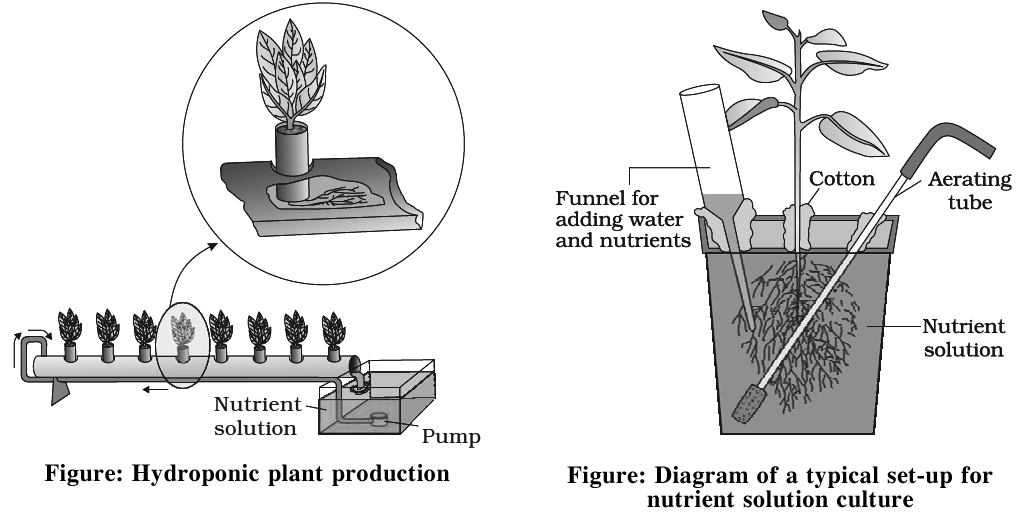
Hydroponics
Commercial technique of soil less culture is called Hydroponics, which was first developed by Goerick (1940).
In 1860, Julius von Sachs, a German botanist, demons rated for the first time, that plants could be grown to maturity in a defined nutrient solution in complete absence of soil.
Culture is performed in large tanks of metal or Reinforced Cement Concrete (R.C.C.) Tanks are covered with wire mesh.
Tanks are provided with aerating and circulating techniques.
Seeds are suspended in solution from the wire mesh with the help of threads.
As plant grows up additional support is provided.
Significance
(i).Useful in areas having thin, infertile and dry soils.
(ii).It can regulate the pH at optimum for a particular crop.
(iii).It controls soil borne pathogens.
(iv).It avoids problem of weeding.
(v).Out of season vegetables (like tomato, seedless cucumber, lettuce) and flowers can also be obtained.
Aeroponics
It is technique of soil-less culture in which roots of plants are suspended in mist of oxygenated nutrient solution.
Sand Culture
In this method, sand is used as a rooting medium and nutrient solution is added to it. It is better than solution cultures w.r.t. providing solid medium and natural aeration for plant. growth. However, this method has following drawbacks:
(i).The sand being highly alkaline in nature, has to be treated with acid before use.
(ii).The sand get very warm during summer and very cool during winters, hence may cause injury to the root system.
(iii).The water holding capacity of sand is very low, hence, it requires freqent watering.
mineral elements that are essential
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Mineral elements that are essential
The inorganic nutrients are classified as essential elements and non essential elements.
17 elements have been placed under essential elements.
These are the elements without which the reproduction and life cycle of a plant cannot be completed.
The essential elements are : C, H, O, N, P, K, S, Mg, Ca, Fe, Mo, Mn, Ni, Zn, B, Cl, Cu.
Essentiality of Minerals
A variety of mineral elements are present in the soil but all of them are not essential for plant growth.
Besides, a particular element may be needed for the growth of one plant and may not be required at all by other plants.
For example, sodium is required in very small amount by the desert shrub Atriplex, but is not required by most of the other plants.
Following criteria given by D.I. Arnon and P.R.Stout (1939) are used to determine essentiality of minerals:
1.The element must be absolutely necessary for normal growth and reproduction. The plant do not complete its life cycle or set the seed in the absence of that particular element
2.The element must not be replaceable by another element.
3.The element must play a direct role in the metabolism of plant.
4.Absence of a specific element causes deficiency in the plant which is corrected only by adding the specific mineral in the soil.
Types of Essential Elements
On the basis of concentration in plant, Hoagland divided essential elements into two groups.
(i) Macronutrients : These are present in more concentration like 1-10 mg per gram of dry weight. These are easily detectable. e.g., C, H, O, N, P, S, Ca, K, Mg.
(ii) Micronutrients : These elements occur in plant body in concentration of equal or less than 0.1 mg per gram of dry weight. Infact these are required in traces, so called trace elements. e.g., Mo, Mn, Zn, B, Cu, Cl, Fe, Ni.
In addition to the 17 essential elements, there are some beneficial elements such as sodium silicon, cobalt and selenium. They are required by higher plants.
General Functions of Mineral Elements
(a) Frame work elements – Form carbohydrates which form cell wall, e.g., C, H, O.
(b) Protoplasmic elements – Form protoplasm, e.g., C, H, O, N, P, S.
(c) Catalytic elements – e.g., Fe, Cu, Zn, Mo, Mg, Mn, K (activator of over 40 enzymes)
(d) Balancing element – Ca, Mg and K counteract the toxic effect of other minerals.
(e) Storage elements – C, N, S, P.
(f) Critical elements – N, P, K.
(g) Minerals influence OP and TP.
(h) Monovalent cations (Na+, K+) Increases permeability of membrane, while divalent and trivalent ions decrease it.
(i) Toxic elments e.g., Al, As, Hg, Pb, Ag.
(j) Non mineral elements e.g., C, H, O, N. N is both mineral and non mineral.
(k) Functional elements: They are non essential in most plants but have a definite activity in some species e.g., silicon in grasses.
absorption of elements
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Absorption of elements
Minerals are mainly absorb by the root which is in direct contact with the soil solution.
Maximum mineral absorption occurs through zone of cell elongation.
The process of absorption can be demarcated into two main phases.
In the first phase, an initial rapid uptake of ions into the 'free space' or 'outer space' of cell -the apoplast, is passive.
In the second phase of uptake, the ions are taken in slowly into the 'inner space' –the symplast of the cells.
The symplast movement requires metabolic energy, i.e., it is an active process.
The movement of dissolved substances into and out of cell is called transport or flux.
Many theories have been given to explain the mechanism of mineral salt absorption.
These theories can be grouped into following two categories:
(1) Passive mineral absorption
(2) Active mineral absorption
(1) Passive absorption
Absorption of ions without use of metabolic energy is known as passive absorption.
Molecules or ions diffuse from a region bf their higher concentration to a region of their lower concentration.
The movement of mineral ions into root cells as a result of diffusion is called passive absorption.
Main theories for passive absorption are described below:
(a) Ion exchange: This theory was proposed by Jenny and Overstreet (1938). Exchange of anions and cations absorbed on colloidal fraction of the soil (clay and humus) with the ions adsorbed on root surface is referred to as ion exchange.
(i) Contact Exchange : This is based on the ion exchange from one adsorbent to another without the participation of free electrolyte. An ion which is adsorbed electrostatically to a solid particle is not tightly bound, but oscillate within a small volume of space. This is termed oscillation volume. According to this concept, H+ ions exchange with the cations and OH– ions exchange with anions.
(ii) Carbonic acid exchange: CO2 is released by root respiration, which forms carbonic acid when dissolved in soil water. This carbonic acid dissociate into H+ and HCO3– ions. Released H+ ions exchange with cations and HCO3– ions exchange with anions.
(b) Donnan equilibrium: This mechanism was proposed by Donnan (1911). Entry of ions into the cell across the plasma membrane to maintain electrical equilibrium is called Donnan equilibrium. Some anions or cations get firmly attached to the inner surface of plasma membrane (fixed and non-diffusible ions). To neutralise these, ions of opposite charges gain entrance in the cell passively (against concentration gradient i.e., without energy expenditure).
(c) Mass flow or Bulk Flow Theory. According to Hylmo, the ion absorption increases with increase in transpiration. The ions have been considered to move in mass with flow of water from the soil solution through the root and eventually to the shoot.
Active mineral absorption
The absorption of ions, involving use of metabolic energy is called active absorption. This occurs against the concentration gradient. Energy used in this mechanism comes from metabolic activities, especially respiration.
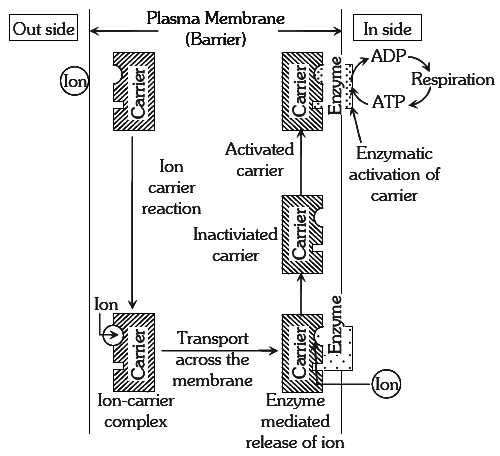
(a) Carrier Concept : This concept was proposed by Van den Honert. According to this concept, there are separate protein carriers for cations and anions. The carrier forms an ion-carrier complex on the outer face of the membrane. This complex dissociates and releases ions into inner-space. The inactivated carrier is again activated by the enzyme kinase. In this process ATP is used up, this activated carrier again accepts new ions and entire cycle is repeated.
(b) Cytochrome Pump Hypothesis - It was proposed by Lundegardh and Burstrom. This gates that anions are absorbed actively and cations passively. At the outer surface of membrane, cytochrome loses an electron durin oxidation and picks up an anion in exchange. It is then transported to the inner side of the membrane through the-cytochrome chain. The cations move passively along the electrical gradient created by the accumulation of anions at the inner surface of membrane. The increased rate of respiration upon anion intake is called as salt respiration.
(c) Protein Lecithin Theory : Proposed by Bennet and Clark. They observed that a phospholipid called lecithin is involved .in transport of ions and act as carrier. The lecithn is composed of phosphatidic acid and choline. Phosphate group in phosphatidic acid is regarded as e active cation binding center and choline is anion binder. These ions are liberated on the inner surface of the membrane by catalysis of lecithin presence of enzyme lecithinase. The regeneration of lecithin from phosphatidic acid and choline occurs in presence of choline acetylase, choline esterase and ATP.
translocation of solutes
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Translocation of solutes
Prerequisites:
Knowledge about methods to study the mineral requirements of plants.
Knowledge of essential elements, their roles and mechanism of absorption of mineral elements in plants.
Concept:
By radio-isotopes, it has been proved that inorganic substances move up the plant through xylem. These substances move along with water by transpiration pull.
The rate at which inorganic solutes are translocated through xylem corresponds to the rate of translocation of water. After absorption of minerals by roots, ions are able to reach xylem by two pathways apoplast and symplast pathway.
soil contains essential elements
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Soil contains essential elements
Prerequisites:
Knowledge about methods to study mineral requirement in plants.
Knowledge of essential elements and their roles in the plant body.
Basic concept about mechanism absorption of elements.
Concept:
Soil provides anchorage, air, water and minerals to the plants growing in it.
Majority of the nutrients that are essential for the growth and development of plants become available to the roots due to weathering and breakdown of rocks. These processes enrich the soil with dissolved ions and inorganic salts. Since they are derived from the rock minerals, their role in plant nutrition is referred to as mineral nutrition.
Soil consists of a wide variety of substances. Soil not only supplies minerals but also harbours nitrogen-fixing bacteria, other microbes.
Since deficiency of essential minerals affect the crop-yield, there is often a need for supplying them through fertilizers.
Both macro-nutrients (N, P, K, S, etc.) and micro-nutrients (Cu, Zn, Fe, Mn, etc.) form components of fertilizers and are applied as per need
Metabolism of Nitrogen
- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Nitrogen is the most prevalent element in living organisms.
The atmosphere contain near about 78% of N2 by volume.
Plants compete with microbes for the limited nitrogen that is available in soil.
Thus, it is a limiting nutrient for both natural and agricultural ecosystems.
N2 cycle can be conveniently discussed under the following steps
(i) N2 fixation (ii) Ammonification (iii) Nitrification (iv) Denitrificatio
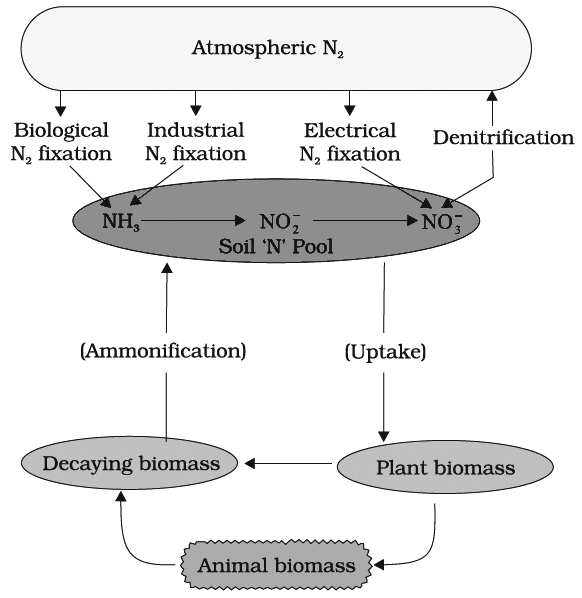
(I) NITROGEN FIXATION
Nitrogen exists as two nitrogen atoms joined by a very strong triple covalent bond (N º N).
The process of conversion dinitrogen (N2) to ammonia is termed as nitrogen fixation.
Following are the methods of N2-fixation:
A. Physico-Chemical method : During thunder, lightening and by using UV rays, atmospheric N2 and oxygen combine to form oxides of nitrogen which form nitrous and nitric acid with water. This may form nitrates of calcium, potassium and ammonium.
B. Industrial N2 fixation: Industrial combustions, forest fires, automobile exhausts and power generating stations are also sources of atmospheric nitrogen oxides.
C. Biological N2 fixation : Only certain prokaryotic species are capable of fixing N2. Biological N2 fixation may be asymbiotic, symbiotic or through loose symbiosis. Biological N2 fixation is called diazotrophy and agents of this process are called diazotrophs.
Some important N2 fixing organisms
(a) Asymbiotic N2 fixers:
Bacteria
(i) Aerobic – Azotobacter, Beijerinckia
(ii) Facultative Aerobic – Klebsiella, Bacillus
(iii) Anaerobic – Clostridium
(iv) Photosynthetic – Chromatium, Rhodospirillum
Blue Green Algae – Anabaena, Aulosira, Nostoc, Scytonema etc. Heterocyst is present in these blue green algae which is responsible for N2 fixation
(b) Symbiotic N2 fixers:
(i) In root nodule of legumes – Rhizobium
(ii) In root nodule of Alnus, Casuarina, Myrica – Frankia
(iii) In leaf nodule of Dioscorea, Pavetta and Psychotria – Klebsiella
(iv) In coralloid root of Cycas – Anabaena cycadae
(v) In fronds of Azolla – Anabaena azollae
(vi) In thallus of Anthoceros – Nostoc
(c) Intermediate: Loose symbiosis with the roots of Sorghum, Zea etc. by Azospirillum.
Rhizobium -Legume Symbiosis
Principal stages of nodule formation are summarised as follows:
1. Rhizobia are Gram negative aerobic rod-shaped bacteria. This genus is responsible for symbiotic N2 fixation in legumes
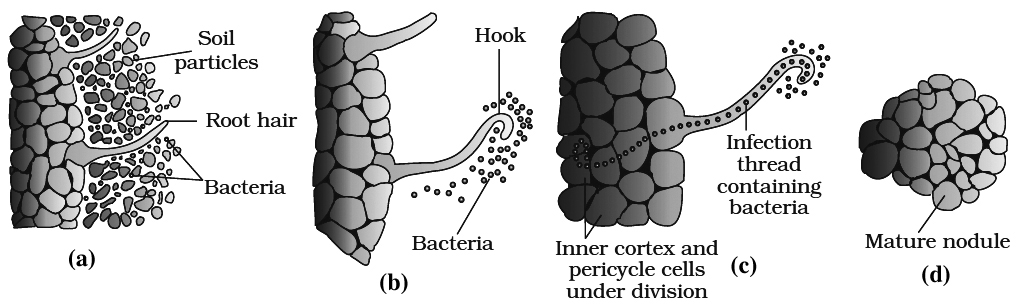
2.Legume roots secrete some specific chemicals (e.g., Flavinoids) which attract the bacteria. Rhizobia multiply an colonise-the surroundings of roots and get attached to epidermal root hair cells.
3.The root hairs curl by the action of nod factors secreted by bacteria and the bacteria invade the root hair.
4.An infection thread is produced, carrying the bacteria into the cortex region of root.
5.Cortical cells are stimulated divide rapidly. It is due to auxins secreted by plants and cytokinins secreted by bacteria.
6.Bacteria enters only polyploid cells of cortex. Some of them enlarge and become membrane bound structures called bacteroids. These form the seat of N2 fixation. These specialised cortical cells now form nodules. Nodules establish a direct vascular connection with the host for exchange of nutrients.
7.The nodules contain a red coloured pigment called leghaemoglobin (LHb). The globin part of leghaemoglobin is formed by host genome, while the heme portion is formed by bacteria.
8.This pigment is O2 carrier and is also called scavenger of O2.
9. Nitrogenase enzyme (synthesized by nif genes of bacteria) is required to fix N2. It is an O2 sensitive enzyme made up of two unequal sub units. Large component has Fe-Mo moiety, while, small component has only Fe-moiety. Here Mo acts as an acceptor and donor of electrons, when N2 is reduced to NH3 LHb maintains anaerobic conditions.
10.N2 fixation requires energy, so it is an active process.
N2 + 8e– + 8H+ + l6 ATP —® 2NH3 + H2 + 16ADP + 16Pi
11.N2 fixation occurs under the controJ of plant nod gene and bacterial nod, nif and fix gene cluster.
12.During this process, atmospheric N2 is reduced by the addition of hydrogen atom.
13.Strong reducing agents e.g., NADPH2, FMNH2, Ferredoxin are also required.
14.Donor of electron and H+ is generally glucose-6-phosphate; certain cofactors like -TPP, Mg++ and CoA are also involved.
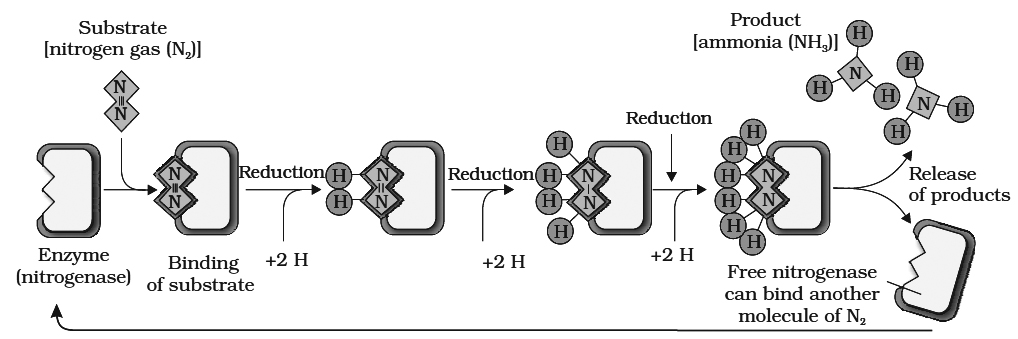
15. ATP is provided by the host respiration process.
16.NH3 so formed is used for the synthesis of amino acids. These acts as building blocks for the synthesis of various types of protein.
(II) AMMONIFICATION
Plants absorb inorganic nitrogen and convert it into proteins
After the death of organisms and plants, proteins are broken into ammonia by the following two steps:
(a) Proteolysis: It is breakdown of protein
![]()
(b) Deamination : Ammonia is released from the amino acids.
Amino acid + H2O ![]() organic acid + ammonia
organic acid + ammonia
It is done by Bacillus ramosus, B. vulgaris, and B. mycoides. This ammonia is converted into nitrate which is absorbed by' the plants.
(III) NITRIFICATION
It is oxidation of ammonia into nitrate, it involves following steps:
(a) Conversion of ammonia into nitrate
2NH3 + 3O2![]() 2NO2– + 2H+ + 2H2O + Energy
2NO2– + 2H+ + 2H2O + Energy
(b) Conversion of nitrite into nitrate.
2NO2– + O2 ![]() 2NO3– + energy
2NO3– + energy
Nitrate assimilation : Nitrate cannot be used by the plant as such. It is first converted into ammonia before being incorporated into organic compounds. Nitrate is reduced in two steps -
The process of nitrate reduction to ammonia is called nitrate assimilation and is accomplished in two steps mediated by two specific enzymes:
(a) First, the nitrate is reduced to nitrite by an enzyme called nitrate reductase. This enzyme is a flavoprotein and contains molybdenum.
(b) The nitrite ions are then reduced to ammonia by an enzyme called nitrite reductase. Ferredoxin is the most direct source of electrons for nitrite reduction and hence, it occurs specifically in leaves. Therefore, nitrite ions formed in other parts of the plant are tranported to leaves and further reduced to ammonia. Nitrite reductase does not require molybdenum but contains copper and iron.
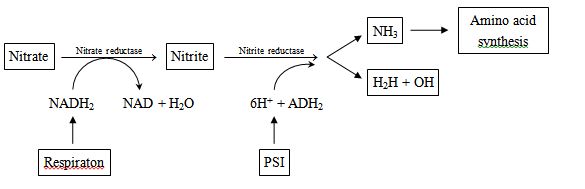
Ammonia thus formed, is fixed by the organic acids to produce amino acids which form other nitrogenous compounds.
(IV) DENITRIFICATION
Nitrates are broken down into gaseous nitrogen or nitrolls oxides by some microorganisms.
It is called denitrification e.g., Thiobacillus denitrificans, Micrococcus denitrificans, Pseudomonas denitrificans.
FATE OF AMMONIA
At physiological pH, the ammonia is protonated to form NH4+ ions, which is quite toxic to plants and hence cannot be accumulated by them. This is commonly used up to synthesize
(a) Amino acids and or (b) Amides
(a) Amino Acids
Amino acids are generally the initial products of N2 assimilation. Mainly, these two processes are used by plants to synthesise amino acid :
(i) Reductive Amination : In this process, NH3 reacts with a-ketoglutaric acid and forms glutamic acid.
a-Ketoglutaric acid + NH4+ + NAD(P)H Glutamic acid + H2O + NAD (P).
(ii) Transamination : It involves the transfer of amino group from one amino acid to the keto group of other keto acid.
Glutamic acid is the main amino acid from which other 17 amino acids are formed through transamination. Enzyme required for this reaction is transaminase.
(b) Amides
Two most important amides present in plants are asparagine and glutamine.
These are formed from two amino acids, glutamic acid and aspartic acid.
For this reaction, glutamine synthetase and asparagine synthetase are required respectively.
Amides contain more N2 than amino acids and are structural part of most proteins, these are transported through xylem vessels.
Inaddition, along with the transpiration stream the nodules of some plants (e.g., soyabean) export the fixed nitrogen as ureides (allantoin, allantoic acid and citrulline).
Important definitions/ formulae:
Some important N2 fixing organisms
(a) Asymbiotic N2 fixers:
Bacteria
(i) Aerobic – Azotobacter, Beijerinckia
(ii) Facultative Aerobic – Klebsiella, Bacillus
(iii) Anaerobic – Clostridium
(iv) Photosynthetic – Chromatium, Rhodospirillum
Blue Green Algae – Anabaena, Aulosira, Nostoc, Scytonema etc. Heterocyst is present in these blue green algae which is responsible for N2 fixation.
(b) Symbiotic N2 fixers:
(i) In root nodule of legumes – Rhizobium
(ii) In root nodule of Alnus, Casuarina, Myrica – Frankia
(iii) In leaf nodule of Dioscorea, Pavetta and Psychotria – Klebsiella
(iv) In coralloid root of Cycas – Anabaena cycadae
(v) In fronds of Azolla – Anabaena azollae
(vi) In thallus of Anthoceros – Nostoc
(c) Intermediate: Loose symbiosis with the roots of Sorghum, Zea etc. by Azospirillum.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
