1.Chemical Equations
Chemical change – one or more new substances with new physical and chemical properties are formed.
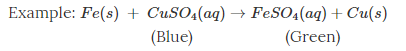
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper are formed.
1. The Chemical Properties of Acids and Bases

Gasjhjashdjashjkhasiwieowqjkehqwjkehjkqwehjkqwhejkqwhejk hhjkdhasjkdhjksahdjhadjkascbnzxcnvznxcvbncvbncvbnzxvcnvcbnzvcbncbncvnbcbzxvcwqeiyqwieyqwuieyqwuieyiqwueyyeiuye whejjwqhejkqwhkjhdjkasdbnasbdnbasdnbasjhdadhjkashdjhasjkdhqwyewqehjkwhjk djhasjhdmasbmnbxmzbbncmnbxcnbdsnmdbfnmbadf ehwrhewjhrjwehrjhwe
5656a565a65d65a67s5dgsvcbvcnvsJDKSLJDFKLJSDKLFJDSKLFJLKVMNVMNV86765664564654$$#!#@$%$^%*&^*(&*(&*^&%^#%$#$@

2.Types of Chemical Reactions
Notes by teacher
3.Effects of Oxidation Reactions in Everyday Life
note by teacher
Sagar Daksh Science Book > Unit I: Chemical Substances - Nature and Behaviour > Chapter 1 Chemical reactions > Implications of a balanced chemical equation.
Types of chemical reactions: combination, decomposition, displacement, double displacement, precipitation, neutralization, oxidation and reduction.
sss

 Pragati
Pragati
