1.Chemical Equations
Chemical change – one or more new substances with new physical and chemical properties are formed.
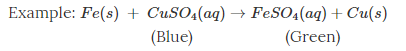
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper are formed.
1.Chemical Equations
A chemical equation is a statement or representation that describes a chemical reaction in terms of
symbol and formulae.
➢ A chemical reaction is the transformation of chemical substance called reactants into another chemical
substance called products. In a chemical reaction, only rearrangement of atoms takes place.
➢ The substances which take part in a chemical reaction are called reactants. The reactants are written
on the left hand side. The new substances produced as a result of chemical reaction are called
products. The products are written on the right hand side.
Some of the symbols used in a chemical Equation are :
(a) Solids (s)
(b) Liquids (l)
(c) Gases (g)
(d) Aqueous solutions (aq)
(e) Gas released as a product (↑)
(f) Precipitate formed in the reaction (↓)
(g) Direction of reaction (→)
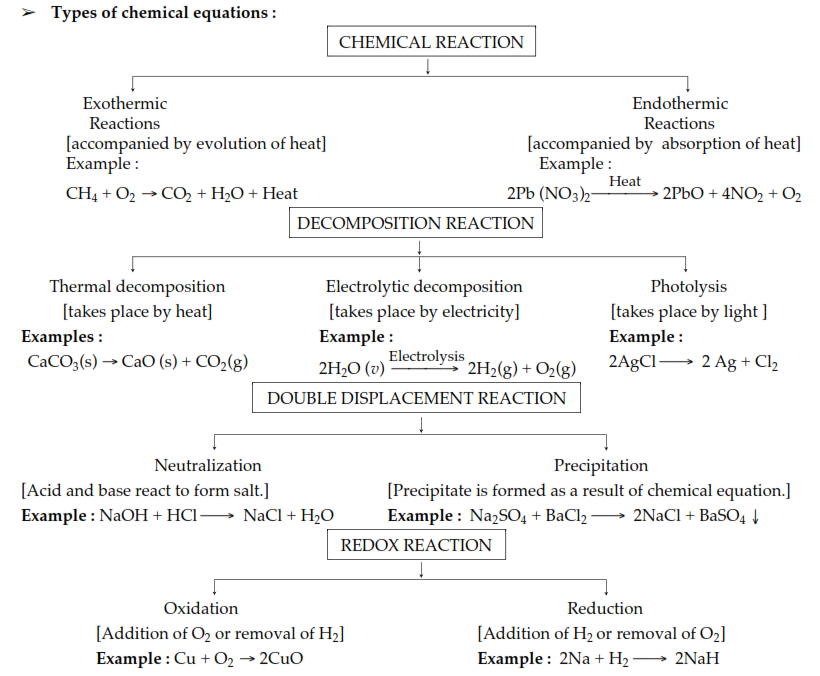
➢ Steps to balance a chemical equation :
(a) Write word equation.
(b) Then write skeletal chemical equation.
(c) Enclose the formula in the boxes.
(d) List the number of atoms of different elements present in the unbalanced equation.
(e) Start balancing with the compound that contains the maximum number of atoms.
(f) Start balancing other atoms.
(g) Check the correctness of the balanced equation.
1.Chemical Equations
- Books Name
- SonikaAnandAcademy Science Book
- Publication
- SonikaAnandAcademy
- Course
- CBSE Class 10
- Subject
- Science
NCERT Solutions -Class 10 -Chemical Reactions and Equations
Chemical Reaction
When two or more substance react with each other to form one or more new substance this is called chemical reaction
The substances which reacts with each other in a chemical reaction are known as reactants they are generally written on the left hand side of the equation
The substances which are formed in a chemical reaction are known as product they are generally written on the right hand side of the equation
Chemical reactions can be of different types
1) Combination reaction-- when two or more substances combine together to form a new substance is called combination reaction
Symbolically
A+B ------C
Two substances A and B are combined together to form a new substance C
Example
H2+Cl2------2HCl
2) Decomposition reaction-- when a substance decomposes into two or more substances is called decomposition reaction
Symbolically
C---A+B
The compound C decomposes to two compounds A and. B
Example
ZnCO3------Zn + CO2
The composition of a compound can be done by either of the methods
--- by thermal decomposition
--- by electrolysis
-----by photolysis reaction
3) Displacement Reaction
When an element of a compound displaces by another element during a chemical reaction is known as displacement reaction
Symbolically
AB+C-----AC+B
In above equation element C displaces element B of the compound AB so this type of reaction is known as displacement reaction
2HCl+Mg------MgCl2 + H2
4) Double displacement reaction
When elements of compounds and exchanged each other in a chemical reaction what is known as double displacement reaction
Symbolically
AB+CD-----AD+BC
In the above reaction elements of the compound AB and elements of the compounds CD are exchange together to form a compounds AD and BC
2KBr+BaI2-------2KI. + BaBr2
Types of reactions positive ion of one compound reacts with negative ions of another compound similarly in negative ions of the first compound combines with positive
Redox Reaction
The reaction in which there is a oxidation and reduction both occurs is known as red oxide reaction in this type of reaction there is gain of electron in one element and loss of electrons in an atom
The element which gains electrons undergoes oxidation reaction it can also be defined as addition of oxygen is known as oxidation reaction
The element which loses electron undergoes reduction reaction or in other words addition of hydrogen is known as reduction reaction
The substance which undergoes oxidation is known as reducing agent
The substance which undergoes reduction is known as oxidising agent
Limiting Reagent
The substance which consumes completely in a chemical reaction is called limiting reagent generali on limiting reagent decide the extent of a chemical reaction
1.Chemical Equations
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 1
Chemical reactions and Equations
Chemical Reaction: The transformation of chemical substance into another chemical substance is known as Chemical Reaction. For example: Rusting of iron, the setting of milk into curd, digestion of food, respiration, etc.
In a chemical reaction, a new substance is formed which is completely different in properties from the original substance, so in a chemical reaction, a chemical change takes place. Only a rearrangement of atoms takes place in a chemical reaction.
The substances which take part in a chemical reaction are called reactants.
The new substances produced as a result of a chemical reaction are called products.
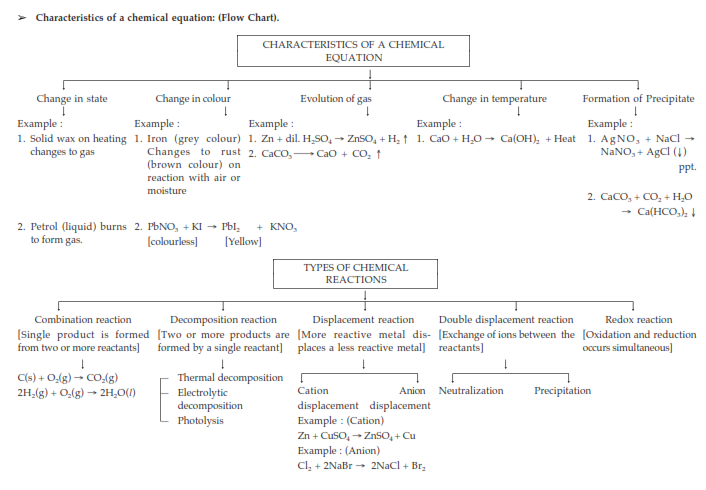
Characteristics of Chemical Reactions:
(i) Evolution of gas: The chemical reaction between zinc and dilute sulphuric acid is characterised by the evolution of hydrogen gas.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) ↑
(ii) Change in Colour: The chemical reaction between citric acid and purple coloured potassium permanganate solution is characterised by a change in colour from purple to colourless.
The chemical reaction between sulphur dioxide gas and acidified potassium dichromate solution is characterized by a change in colour from orange to green.
(iii) Change in state of substance: The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas (because the wax is a solid, water formed by the combustion of wax is a liquid at room temperature whereas, carbon dioxide produced by the combustion of wax is a gas). There are some chemical reactions which can show more than one characteristic.
(iv) Change in temperature: The chemical reaction between quick lime water to form slaked lime is characterized by a change in temperature (which is a rise in temperature).
The chemical reaction between zinc granules and dilute sulphuric acid is also characterised by a change in temperature (which is a rise in temperature).
(v) Formation of precipitate: The chemical reaction between sulphuric acid and barium chloride solution is characterised by the formation of a white precipitate of barium sulphate.
BaCl2(aq) + H2SO4(aq) → BaSO4(s) (ppt) + 2HCl(aq)
Chemical Equation:
Representation of chemical reaction using symbols and formulae of the substances is called Chemical Equation.
Example: A + B → C + D
In this equation, A and B are called reactants and C and D are called the products. The arrow shows the direction of the chemical reaction. Condition, if any, is written generally above the arrow.
When hydrogen reacts with oxygen, it gives water. This reaction can be represented by the following chemical equation:
Hydrogen + Oxygen → Water H2 + O2 → H2O
In the first equation, words are used and in second, symbols of substances are used to write the chemical equation. For convenience, the symbol of substance is used to represent chemical equations.
A chemical equation is a way to represent the chemical reaction in a concise and informative way.
A chemical equation can be divided into two types: Balanced Chemical Equation and Unbalanced Chemical Equation.
(a) Balanced Chemical Equation: A balanced chemical equation has the number of atoms of each element equal on both sides.
Example: Zn + H2SO4 → ZnSO4 + H2
In this equation, numbers of zinc, hydrogen and sulphate are equal on both sides, so it is a Balanced Chemical Equation.
According to the Law of Conservation of Mass, mass can neither be created nor destroyed in a chemical reaction. To obey this law, the total mass of elements present in reactants must be equal to the total mass of elements present in products.
(b) Unbalanced Chemical Equation: If the number of atoms of each element in reactants is not equal to the number of atoms of each element present in the product, then the chemical equation is called Unbalanced Chemical Equation.
Example: Fe + H2O → Fe3O4 + H2
In this example, a number of atoms of elements are not equal on two sides of the reaction. For example; on the left-hand side only one iron atom is present, while three iron atoms are present on the right-hand side. Therefore, it is an unbalanced chemical equation.
Steps to form balanced chemical equation
To show how to balance the equation, the following equation is used-
Fe + H2O → Fe3O4 + H2
Step 1: First of all, draw the boxes around each formula as shown below-
![]()
Step 2: Find out the number of atoms of each element. For Example, on reactant side, 1 for Fe, 2 H, and 1 O and on product side we have, 3 for Fe, 4 for O and 2 for H.
Step 3: Start to balance the equation with the compound having maximum number of atoms. While balancing does not alter the formula of the compound.
Step 4: One by one balance each element on reactant and product side.
![]()
Step 5: After balancing number of atoms on both the side of the equation, finally check the correctness of the balanced equation.
![]()
Step 6: then write the symbols of the physical state of reactants and products as shown below-
3Fe(s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g)
This above equation represents the balanced equation.
1. The Chemical Properties of Acids and Bases

Gasjhjashdjashjkhasiwieowqjkehqwjkehjkqwehjkqwhejkqwhejk hhjkdhasjkdhjksahdjhadjkascbnzxcnvznxcvbncvbncvbnzxvcnvcbnzvcbncbncvnbcbzxvcwqeiyqwieyqwuieyqwuieyiqwueyyeiuye whejjwqhejkqwhkjhdjkasdbnasbdnbasdnbasjhdadhjkashdjhasjkdhqwyewqehjkwhjk djhasjhdmasbmnbxmzbbncmnbxcnbdsnmdbfnmbadf ehwrhewjhrjwehrjhwe
5656a565a65d65a67s5dgsvcbvcnvsJDKSLJDFKLJSDKLFJDSKLFJLKVMNVMNV86765664564654$$#!#@$%$^%*&^*(&*(&*^&%^#%$#$@

1. The Chemical Properties of Acids and Bases
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 2
Acids, Bases & Salts
Introduction
- A substance that tastes sour in water, turns blue litmus red, and neutralises bases is known as an acid.
- If a substance’s aqueous solution tastes bitter, turns red litmus blue, or neutralises acids, it’s called a base.
- Salt is a neutral material that has no effect on litmus in aqueous solution.
Acids and Bases Reactions
A neutralisation reaction occurs when an acid reacts with a base. A salt and water are the end products of this reaction. An acid–base neutralisation reaction is formulated as a double-replacement reaction in this standard approach.
Reaction of Acid & Bases
a) Reaction of Acid & Bases with Metals
Acids, in general, react with metals to produce salt and hydrogen gas. Bases, in general, do not react with metals and do not produce hydrogen gas.
Acid + active metal → Salt + hydrogen + heat
2HCl + Mg → MgCl2 + H2 (↑)
Hydrochloric acid + Magnesium → Magnesium chloride + Hydrogen
Base + metal → Salt + hydrogen + heat
2NaOH + Zn → Na2ZnO2 + H2 (↑)
Sodium hydroxide + Zinc → Sodium zincate + Hydrogen
A more reactive metal displaces the less reactive metal from its base.
2Na + Mg (OH) 2 → 2NaOH + Mg
Sodium + Magnesium hydroxide → Sodium hydroxide + Magnesium
b) Reaction of Acids with metal carbonates and bicarbonates
Acids produce carbon dioxide, as well as metal salts and water, when they react with metal carbonates or metal bicarbonates. Sodium chloride, carbon dioxide, and water are formed when sodium carbonate interacts with hydrochloric acid. Allowing carbon dioxide gas to travel through lime water turns it milky.
Acid + metal carbonate or bicarbonate → salt + water + carbon dioxide.
2HCl + CaCO3 → CaCl2 + H2O + CO2
H2SO4 + Mg (HCO3)2 → MgSO4 + 2H2O + 2CO2
Effervescence indicates liberation of CO2 gas.
c) Reaction of Acid with base
1. Reaction of metal oxides and hydroxides with acids
Metal oxides or metal hydroxides are basic in nature.
Acid + base → salt + water + heat
H2SO4 + MgO → MgSO4 + H2O
2HCl + Mg (OH) 2 → MgCl2 + 2H2O
2. Reaction of non-metal oxides with bases
Non-metal oxides are acidic in nature
Base + Nonmetal oxide → salt + water + heat
2NaOH + CO2→ Na2CO3 + H2O
3. Reaction of acids and base
A very common acid is hydrochloric acid. The reaction between strong acid says hydrochloric acid and strong base say sodium hydroxide forms salt and water. The complete chemical equation is shown below.
HCl (strong acid) + NaOH (strong base) → NaCl (salt) + H2O (water)
1. Physical Properties of Metals and Non-Metals
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 3
Metals & Non metals
Introduction
Elements are classified as metals and non-metals based on different properties. The properties of metals and non-metals are given in the form of the table below-
Physical Properties of Metals
(i) They are lustrous, i.e., they have a shiny surface.
(ii) All metals are solids except mercury which is liquid at room temperature.
(iii) Metals are ductile which means they can be drawn into wires.
(iv)Metals are malleable which means they can be beaten into sheets. The only exception is mercury.
(v) They are good conductors of heat and electricity except lead and mercury.
(vi)They have high density and high melting point except alkali metals like lithium, sodium, potassium is so soft that they can be cut with a knife.
Physical Properties of Non-metals
(i) They are non-lustrous and have dull appearance except graphite and iodine that are lustrous.
(ii) They are generally soft, except diamond.
(iii) They are non-ductile.
(iv)They are non-malleable.
(v) They are poor conductors of heat and electricity except graphite which is a good conductor of electricity
(vi)They have low density and low melting point except diamond which has high melting point.
1. Bonding in Carbon
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 4
Carbon & its Compounds
Introduction
Covalent Bond: The atomic number of carbons is 6. Its electronic configuration is 2, 4. It requires, 4 electrons to achieve the inert gas electronic configuration. But carbon cannot form an ionic bond.
It could gain four electrons forming C4- cation. But it would be difficult for the nucleus with six protons to hold on to ten electrons.
It could lose four electrons forming C4+ cations. But it requires a large amount of energy to remove four electrons.
Thus, carbon overcomes this problem by sharing of its valence electrons with other carbon atoms or with atoms of other elements.
The bond formed by mutual sharing of electron pairs between two atoms in a molecule is known as Covalent Bond.
Types of Covalent Bond:
Single Covalent Bond: When a single pair of electrons are shared between two atoms in a molecule. For example; F2, Cl2, H2 etc.
The atomic number of hydrogen is 1. Hence it has one electron in its K shell and it requires one more electron to fill the K shell. So, two hydrogen atoms share their electrons to form a molecule of hydrogen, H2.
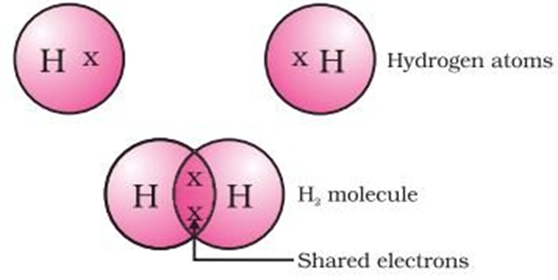
Double Covalent Bond: When two pairs of electrons are shared between two atoms in a molecule. For example; O2, CO2 etc.
The atomic number of oxygen is 8. So it has six electrons in its L shell and it requires two more electrons to complete its octet. So each atom of oxygen shares two electrons with another atom of oxygen. The two electrons contributed by each oxygen atom give rise to a double bond between the two atoms.
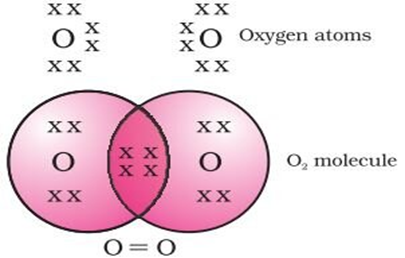
Triple Covalent Bond: When three pairs of electrons are shared between two atoms in a molecule. For example; N2 etc.
Nitrogen has the atomic number 7. So, it has five electrons in its L shell and it requires three more electrons to complete its octet. So, in order to attain an octet, each nitrogen atom in a molecule of nitrogen contributes three electrons giving rise to three shared pairs of electrons that results in a triple bond between the two atoms.
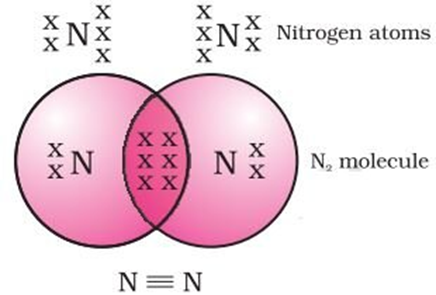
Allotropes of Carbon
- Different forms of an element that have same chemical properties but different physical properties are known as Allotropes.
- There are three allotropes of carbon - Diamond, graphite and Fullerene
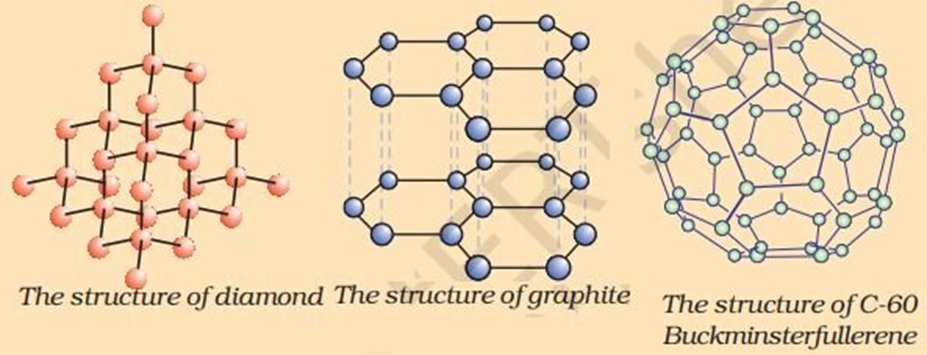
(i) Diamond
In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
→ It is the hardest substance known.
→ It has high melting point.
→ It is a bad conductor of electricity.
→ It is used in making jewellery. It is also used in cutting and drilling tools.
(ii) Graphite
→ Each carbon atom is bonded with other three carbon atoms in the same plane forming hexagonal rings.
→ These rings are stacked over each other through weak Van der Waals forces.
→ Graphite is also a very good conductor of electricity.
→ It is used as dry lubricant.
→ It is used in making pencil lead.
(iii) Fullerene
→ C60, also known as Buck minster fullerene, is the most common form of fullerenes.
→ It has carbon atoms arranged in the shape of a football.
→ It consists of 60 carbon atoms arranged in 12 pentagons and 20 hexagons.
Versatile nature of carbon
Carbon is included in ten formations of a variety of compounds. This is due to the following two reasons:
(i) Catenation: Carbon has the unique ability to form covalent bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation.
Carbon exhibits catenation property due to the following reasons:
→ Size of the carbon atom is very small. This enables its nucleus to hold the shared pair of electrons strongly.
→ The carbon-carbon bond is quite strong.
(ii) Tetravalent Nature: Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
1. Early Classification of Elements
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 5
Periodic Classification of Elements
Introduction
At present, 118 elements are known to us.
All these have different properties. Out of these 118, only 98 are naturally occurring.
These elements have different characteristic properties, so it is very difficult to study these elements individually.
Dobereiner’s Triads (1817):
He identified some triads (groups having three elements).
Dobereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was the average of the atomic masses of the other three elements. For example, Dobereiner could identify only three triads from the elements known at that time. Hence, this system of classification into triads was not found to be useful.
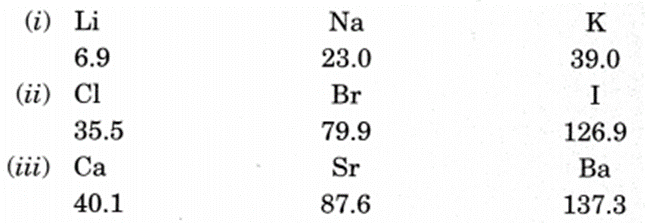
Newland’s Law of Octaves (1866):
- He arranged the known elements in the order of increasing atomic masses.
- He found that every eighth element had properties similar to that of the first like the musical note. It is known as Newlands law of octaves.
Limitations
- Law of octaves was applicable only upto calcium.
- Discovery of Noble gases disturbed the octaves.
- Octaves worked well only for lighter elements.
- Properties of new elements could not fit in it.
2.Types of Chemical Reactions
Notes by teacher
2.Types of Chemical Reactions
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Types of chemical reaction
Combination Reaction is reaction when single product is formed from the combination of two or more reactants. For Example-
![]()
Reactions can be exothermic as well as endothermic.
Exothermic reaction release heats and raises the temperature of the surroundings. For Example, Respiration is an example of exothermic reaction.
Endothermic reaction involved the absorption of the heat and thus it cools the surrounding. The decomposition of dead organic material is an endothermic reaction.
(a) Decomposition Reaction is type of reaction which involves breakdown of single reactant into simpler products. Decomposition of silver chloride into silver and chlorine in presence of sunlight is an example of decomposition reaction.
![]()
(b) Displacement Reaction is a reaction in which more reactive element will displaces the less reactive element.
![]()
(c) Double Displacement Reaction is a type of reaction in which cations and anions in the reactants switch the places to form new products.
![]()
(d) Redox Reaction is also known as Oxidation-reduction Reaction. In this type of reaction, transfer of electrons occurs between the two species. Oxidation is defined as addition of oxygen or removal of hydrogen. Reduction is defined as removal of oxygen or addition of hydrogen. Oxidizing agent is the one which gains the electrons and is reduced in a chemical reaction. Reducing agent is oxidized in a chemical reaction and it loses the electrons. Fluorine is the strongest oxidizing agent. Formic acid is a reducing agent.
![]()
3.Effects of Oxidation Reactions in Everyday Life
note by teacher
Sagar Daksh Science Book > Unit I: Chemical Substances - Nature and Behaviour > Chapter 1 Chemical reactions > Implications of a balanced chemical equation.
3.Effects of Oxidation Reactions in Everyday Life
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Corrosion
Metals are prone to corrosion. It is a slow conversion of metals into some undesirable compounds. This occur may be due to reaction with oxygen, gases, acids etc. When irons react with atmospheric oxygen and moisture, a red layer is formed on the surface of the iron, this process is known as Rusting.
Rancidity
When food containing fats and oils are exposed to the atmosphere, the oxidation of fat and oil occurs, this is known as Rancidity.
Methods to prevent Rancidity
- Store cooking oils from direct sunlight.
- Food should be placed at low temperature.
- By adding antioxidants food can be protected from rancidity.
- Packing material should replace the air with nitrogen.
- Minimize the use of salts in fried foods.
Types of chemical reactions: combination, decomposition, displacement, double displacement, precipitation, neutralization, oxidation and reduction.
sss
2. Common Things in All Acids and Bases
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Acid & bases in water Solution
Dilution of Acid and Base: The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows the concentration of acid or base.
By mixing of acid to water, the concentration of hydrogen ion per unit volume decreases. Similarly, by addition of base to water, the concentration of hydroxide ion per unit volume decreases. This process of addition of acid or base to water is called Dilution and the acid or base is called Diluted.
The dilution of acid or base is exothermic. Thus, acid or base is always added to water and water is never added to acid or base. If water is added to a concentrated acid or base, a lot of heat is generated, which may cause splashing out of acid or base and may cause severe damage as concentrated acid and base are highly corrosive.
Strength of Acid and Base: Acids in which complete dissociation of hydrogen ion takes place are called Strong Acids. Similarly, bases in which complete dissociation of hydroxide ion takes place are called Strong Bases.
In mineral acid, such as hydrochloric acid, sulphuric acid, nitric acid, etc. hydrogen ion dissociates completely and hence, they are considered as strong acids. Since inorganic acids hydrogen ions do not dissociate completely, so they are weak acids.
3. Strongness about Acids or Bases
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Universal indicator
A universal indicator has a pH range from 0 to 14 that indicates the acidity or alkalinity of a solution.
A neutral solution has pH=7
Importance of pH in everyday life
1. pH sensitivity of plants and animals
Plants and animals are sensitive to pH. Crucial life processes such as digestion of food, functions of enzymes and hormones happen at a certain pH value.
2. pH of Soil
The pH of a soil optimal for the growth of plants or crops is 6.5 to 7.0.
3. pH in digestive system
The process of digestion happens at a specific pH in our stomach which is 1.5 to 4.
The pH of the interaction of enzymes, while food is being digested, is influenced by HCl in our stomach.
4. pH in tooth decay
Tooth decay happens when the teeth are exposed to an acidic environment of pH 5.5 and below.
5. pH of self-defense by animals and plants
Acidic substances are used by animals and plants as a self-defense mechanism. For example, bee and plants like nettle secrete a highly acidic substance for self-defense. These secreted acidic substances have a specific pH.
4. About Salts
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Characteristics of salt:
- Most of the salts are crystalline soild.
- Salts may be transparent or opaque.
- Most of the salts are soluble in water.
- Solution of the salts conducts electricity in their molten state also.
- The salt may be salty, sour, sweet, bitter and umami (savoury).
- Neutral salts are odourless.
- Salts can be colourless or coloured.
Family of Salt: Salts having common acidic or basic radicals are said to belong to the same family.
Example:
(i) Sodium chloride (NaCl) and Calcium chloride (CaCl2) belongs to chloride family.
(ii) Calcium chloride (CaCl2) and Calcium sulphate (CaSO4) belongs to calcium family.
(iii) Zinc chloride (ZnCl2) and Zinc sulphate (ZnSO4) belongs to the zinc family.
Neutral, Acidic and Basic Salts:
(i) Neutral Salt: Salts produced because of reaction between a strong acid and strong base are neutral in nature. The pH value of such salts is equal to 7, i.e. neutral.
Example : Sodium chloride, Sodium sulphate. Postassium chloride, etc.
(ii) Acidic Salts: Salts which are formed after the reaction between a strong acid and weak base are called Acidic salts. The pH value of acidic salt is lower than 7. For example Ammonium sulphate, Ammonium chloride, etc.
Ammonium chloride is formed after reaction between hydrochloric acid (a strong acid) and ammonium hydroxide (a weak base).
(iii) Basic Salts: Salts which are formed after the reaction between a weak acid and strong base are called Basic Salts. For example; Sodium carbonate, Sodium acetate, etc.
Sodium carbonate is formed after the reaction between sodium hydroxide (a strong base) and carbonic acid (a weak acid).
Cause of formation of acidic, basic and neutral salts:
- When a strong acid reacts with a weak base, the base is unable to fully neutralize the acid. Due to this, an acidic salt is formed.
- When a strong base reacts with a weak acid, the acid is unable to fully neutralize the base. Due to this, a basic salt is formed.
- When equally strong acid and a base react, they fully neutralize each other. Due to this, a neutral salt is formed.
- Some Important Chemical Compounds
1. Common Salt (Sodium Chloride): Sodium chloride (NaCl) is also known as Common or Table Salt. It is formed after the reaction between sodium hydroxide and hydrochloric acid. It is a neutral salt. The pH value of sodium chloride is about 7. Sodium chloride is used to enhance the taste of food. Sodium chloride is used in the manufacturing of many chemicals.
Important chemical from sodium chloride Sodium Hydroxide (NaOH):
Sodium hydroxide is a strong base. It is also known as caustic soda. It is obtained by the electrolytic decomposition of solution of sodium chloride (brine). In the process of electrolytic decomposition of brine (aqueous solution of sodium chloride), brine decomposes to form sodium hydroxide. In this process, chlorine is obtained at anode and hydrogen gas is obtained at cathode as by products. This whole process is known as Chlor – Alkali process.
Use of products after the electrolysis of brine:
- Hydrogen gas is used as fuel, margarine, in making of ammonia for fertilizer, etc.
- Chlorine gas is used in water treatment, manufacturing of PVC, disinfectants, CFC, pesticides. It is also used in the manufacturing of bleaching powder and hydrochloric acid.
- Sodium hydroxide is used for degreasing of metals, manufacturing of paper, soap, detergents, artificial fibres, bleach, etc.
2. Bleaching Powder (CaOCl2): Bleaching powder is also known as chloride of lime. It is a solid and yellowish white in colour. Bleaching powder can be easily identified by the strong smell of chlorine.
When calcium hydroxide (slaked lime) reacts with chlorine, it gives calcium oxychloride (bleaching powder) and water is formed.
Aqueous solution of bleaching powder is basic in nature. The term bleach means removal of colour. Bleaching powder is often used as bleaching agent. It works because of oxidation. Chlorine in the bleaching powder is responsible for bleaching effect.
Use of Bleaching Powder:
- Bleaching powder is used as disinfectant to clean water, moss remover, weed killers, etc.
- Bleaching powder is used for bleaching of cotton in textile industry, bleaching of wood pulp in paper industry.
- Bleaching powder is used as oxidizing agent in many industries, such as textiles industry, paper industry, etc.
3. Baking Soda (NaHCO3): Baking soda is another important product which can be obtained using by-products of chlor – alkali process. The chemical name of baking soda is sodium hydrogen carbonate (NaHCO3) or sodium bicarbonate.
Properties of Sodium Bicarbonate:
- Sodium bicarbonate is white crystalline solid, but it appears as fine powder.
- Sodium hydrogen carbonate is amphoteric in nature.
- Sodium hydrogen carbonate is sparingly soluble in water.
- Thermal decomposition of sodium hydrogen carbonate (baking soda).
- When baking soda is heated, it decomposes into sodium carbonate, carbon dioxide and water.
2NaHCO3 + heat → Na2CO3 + CO2 + H2O - Sodium carbonate formed after thermal decomposition of sodium hydrogen carbonate decomposes into sodium oxide and carbon dioxide on further heating.
Na2CO3 → Na2O + CO2
This reaction is known as Dehydration reaction.
Use of Baking Soda:
- Baking soda is used in making of baking powder, which is used in cooking as it produces carbon dioxide which makes the batter soft and spongy.
- Baking soda is used as an antacid.
- Baking soda is used in toothpaste which makes the teeth white and plaque free.
- Baking soda is used in cleansing of ornaments made of silver.
- Since sodium hydrogen carbonate gives carbon dioxide and sodium oxide on strong heating, thus, it, is used as a fire extinguisher.
Baking Powder: Baking powder produces carbon dioxide on heating, so it is used in cooking to make the batter spongy. Although, baking soda also produces carbon dioxide on heating, but it is not used in cooking because on heating, baking soda produces sodium carbonate along with carbon dioxide. The sodium carbonate, thus, produced, makes the taste bitter.
Baking powder is the mixture of baking soda and a mild edible acid. Generally, tartaric acid is mixed with baking soda to make baking powder.
When baking powder is heated, sodium hydrogen carbonate (NaHCO3) decomposes to give CO2 and sodium carbonate (Na2CO3). CO2 causes bread and cake fluffy. Tartaric acid helps to remove bitter taste due to formation of Na2CO3.
4. Washing Soda (Sodium Carbonate)
Sodium carbonate is a crystalline solid and it is soluble in water when most of the carbonates are insoluble in water.
Use of sodium carbonate:
- It is used in the cleaning of cloths, especially in rural areas.
- In the making of detergent cake and powder.
- In removing the permanent hardness of water.
- It is used in glass and paper industries.
The water of Crystallization: Many salts contain water molecule and are known as Hydrated Salts. The water molecule present in salt is known as Water of crystallization.
Examples:
Copper sulphate pent hydrate (CuSO4.5H2O): Blue colour of copper sulphate is due to presence of 5 molecules of water. When copper sulphate is heated, it loses water molecules and turns: into grey – white colour, which is known as anhydrous copper sulphate. After adding water, anhydrous copper sulphate becomes blue again.
Plaster of Paris
Plaster of Paris is a widely used chemical compound that is extensively used in sculpting materials and gauze bandages. Plaster of Paris is a white powdery chemical compound that is hydrated calcium sulphate that is usually obtained by calcining gypsum. While we have seen many applications of this material in our everyday lives, if we try to understand its chemistry, we will find that it is a white powdery chemical compound that is hydrated calcium sulphate that is usually obtained by calcining gypsum. To put it another way, Plaster of Paris is often manufactured of heated gypsum at a high temperature.
Gypsum plaster is another name for plaster of Paris. Plaster of Paris is expressed as CaSO4. ½ H2O in chemical formula.
Gypsum, CaSO4.2H2O (s) on heating at 100°C (373K) gives CaSO4. ½ H2O and 3/2 H2O
CaSO4. ½ H2O is plaster of paris.
CaSO4. ½ H2O means two formula units of CaSO4 share one molecule of water.
Uses – cast for healing fractures.
2. Chemical Properties of Metals
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chemical properties of Metals
Metals react with air or oxygen to form metal oxide.
For Example, Copper reacts with oxygen to form copper oxide.
Metal + O2 → Metal oxide
2Cu + O2 → 2CuO
4Al + 3O2 → 2Al2O3
Oxides of metals can react with both acids and bases to produce salt and water. Such oxides are known as Amphoteric Oxides.
Al2O3 + 6HCl → 2AlCl3 + H2O

Metals also react with water to form metal oxide. Metal oxide in turn can react with water to form metal hydroxide.
For Example
2Na + 2H2O → 2NaOH + 1H2
2Al + 3H2O → Al2O3 + 3H2
Metals also react with dilute acids to form salt and hydrogen.
For example, magnesium reacts with dilute hydrochloric acid to form magnesium chloride and hydrogen.
Metal + Acid → Metal Salt + Hydrogen
Mg + 2HCl → MgCl2 + H2
Reaction of metals with solutions of other metal salts
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Fe + CuSO4 → FeSO4 + Cu
Cu + 2AgNO3 → Cu (NO3) + 2Ag
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Fe + CuSO4 → FeSO4 + Cu
Cu + 2AgNO3 → Cu (NO3) + 2Ag
Special cases
- Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
- At ordinary temperature, the surfaces of metals such as magnesium, aluminum, zinc, lead, etc., are covered with a thin layer of oxide. The protective oxide layer prevents the metal from further oxidation.
- Iron does not burn on heating but iron filings burn vigorously when sprinkled in the flame of the burner.
- Copper does not burn, but the hot metal is coated with a black coloured layer of copper (II) oxide.
- Silver and gold do not react with oxygen even at high temperatures.
Chemical properties of Non-metals
Non-metals react with oxygen to form non-metal oxide.
Non-metal + Oxygen → Non-metal oxide
C + O2 → CO2
Non-metals do not react with water and acids to evolve hydrogen gas.
Non-metals can react with salt solution; the more reactive element will displace the less reactive non-metal.
2 NaBr (aq) + Cl2(aq) → 2NaCl (aq) + Br2 (aq)
Non-metals can also react with hydrogen to form hydrides.
H2(g) + S(l) → H2S(g)
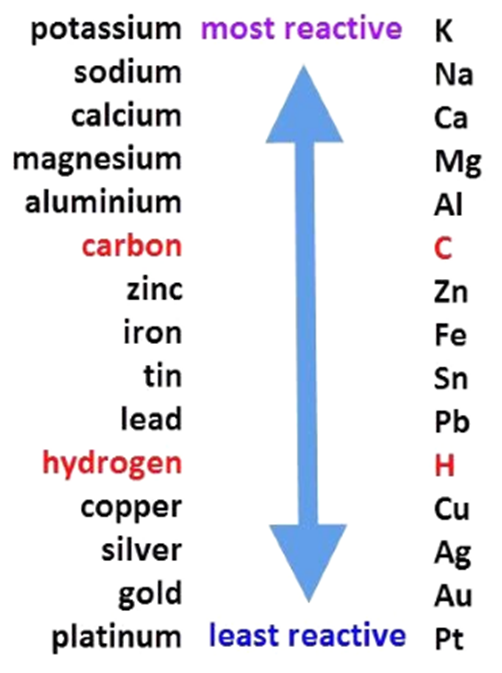
Reactivity Series
The series in which metals are arranged in the decreasing order of reactivity is known as the Reactivity Series.
3. Metals and Non-Metals Reactions
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
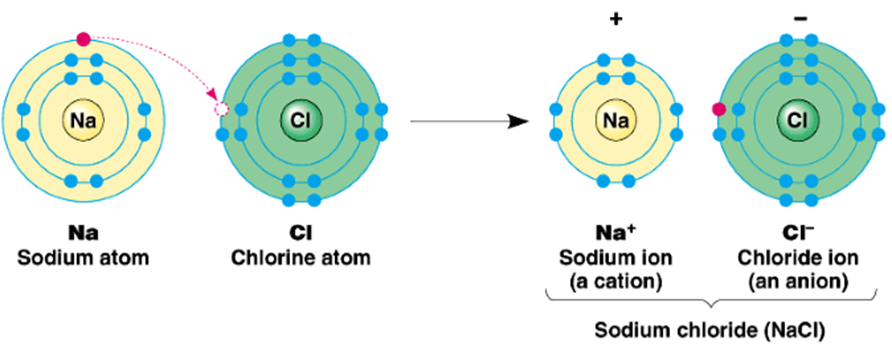
Ionic Compounds
Compounds formed due to the transfer of electrons from a metal to a non-metal are known as Ionic Compounds.
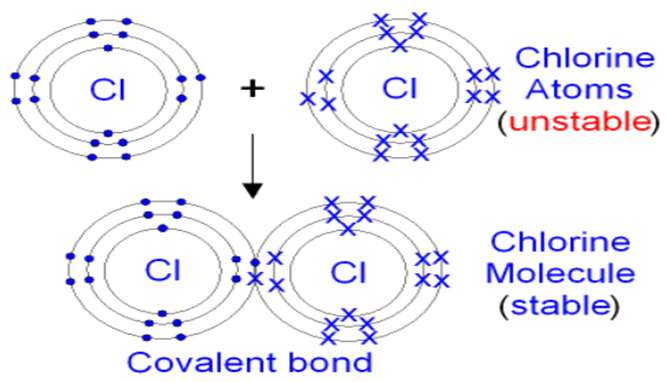
Covalent Bond
A bond is formed by the sharing of electrons between the two atoms. They share their valence electrons to gain stability.
Properties of Ionic Compounds
- They are generally hard and solid.
- They have a high melting and boiling point.
- They are soluble in water but insoluble in inorganic solvents such as ether etc.
- They are conductors of electricity in molten and solution states.
4. occurrence of Metals
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Occurrence of Metals
Metals are generally found in the earth’s crust.
Elements or compounds which occur naturally in earth crust are known as Minerals. Minerals from which pure metals can be extracted are known as Mineral Ores.
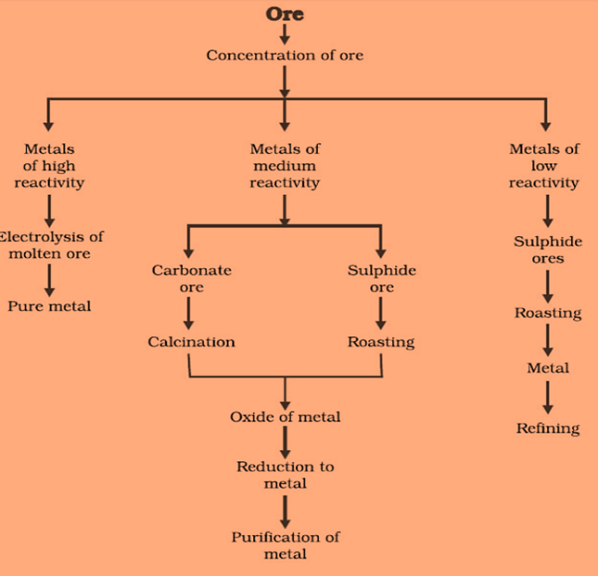
Steps for extraction of metals from its ore are:
- The first step is the enrichment of the ore
- The second step includes extraction of metals
- Third steps involve refining of metal
Gangue - Ores contain different impurities in it such as sand, soil etc. These impurities are known as Gangue.
Extracting Metals which are low in activity series
Metals which are low in the activity series are unreactive. The oxides of such metals can be reduced to metals by heating alone. For Example, Cinnabar (HgS)

Extracting Metals in the middle of the Activity Series
These metals are moderately reactive. They exist as sulphides or carbonates in nature. Before reduction, metal sulphides and carbonates must be converted into metal oxides.by heating strongly in the presence of excess air, this is known as Roasting. Carbonate ores are converted into oxides by heating in limited air. This is known as Calcination.
Roasting

Calcination

Reduction-metal oxides can be reduced to metals using a reducing agent such as Carbon.
Extracting metals towards the top of the activity series
The metals are highly reactive. They cannot be obtained by heating. For Example, Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
At cathode Na+ + e- → Na
At anode 2Cl- → Cl2 + 2e-
Refining of metals
Refining of impure metal is done using electrolytic refining. Impure copper is used as anode and a strip of pure copper is used as Cathode. Acidified copper sulphate is used as an electrolyte. When an electric current is passed through this, impure metal from the anode gets deposited in the electrolyte solution, whereas pure metal from the electrolyte is deposited at the cathode.
Deposition of insoluble residue formed from the dissolution of the anode during commercial electrolysis.
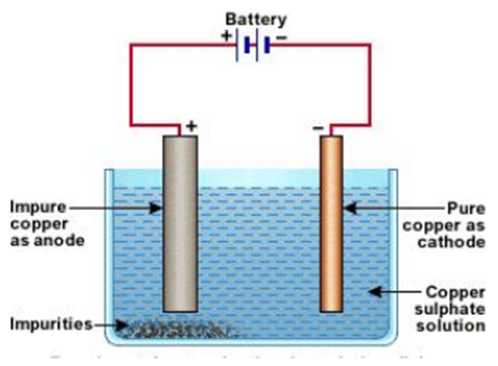
5. Corrosion
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Corrosion
When exposed to moist air for a long period of time, metals become corroded. This is known as Corrosion. For Example, Silver reacts with moist air and becomes black in colour due to silver sulphide coating.
Iron + oxygen → Iron (III) oxide
Fe + O2 → Fe2O3
Prevention of Corrosion
- Rusting of iron can be prevented by oiling, galvanising, painting, greasing etc.
- To protect steel and iron from rusting, a thin layer of zinc is coated on them, this is known as Galvanization.
2. Nature of Carbon Compounds
- Books Name
- SonikaAnandAcademy Science Book
- Publication
- SonikaAnandAcademy
- Course
- CBSE Class 10
- Subject
- Science
Carbon and its compounds
Sample paper
- 1)What is catenation
- 2)What are isomers write the isomers of butane
- 3) What is the mean by homologous series explain with example
- 4) What is the difference between saturated and unsaturated compounds give two examples of each
- 5) How many number of bonds are present in alkane alkene and alkyne
- 6) What is the general formula of alkyl group and cycloalkanes
- 7) Write a formula of
- cyclopentane
- Propyne
- butanol
- Benzene
- 8) What are soaps explain its structure and cleansing action of soap
- 9) what do the detergent forms when they mix and water and what is the difference between detergent and soap
- 10) What are the products formed
- When the flame burns with blue flame and
- when the bus with yellow frame
11)
What is difference between combustion and oxidation
12) what do you mean by saponification explain with reaction
13) what is the eastrification give the chemical reaction and what are the products formed
14) what are addition and substitution reactions give one example of each
15) explain the tetravalency of carbon what is the reason why there are lot of carbon compounds are made by the carbon
2. Nature of Carbon Compounds
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Hydrocarbons
Compounds of carbon and hydrogen are known as hydrocarbons.
For example - Methane (CH4), Ethane (C2H6), etc.
Saturated hydrocarbons: These hydrocarbons have all carbon-carbon single bonds. These are known as alkanes.
General formula = CnH2n+2
where n = number of carbon atoms
Unsaturated hydrocarbons: These hydrocarbons have at least one carbon-carbon double or triple bond.
→ Hydrocarbons with carbon-carbon double bond are known as alkenes. General formula = CnH2n
→ Hydrocarbons with carbon-carbon triple bond are known as alkynes. General formula = CnH2n-2
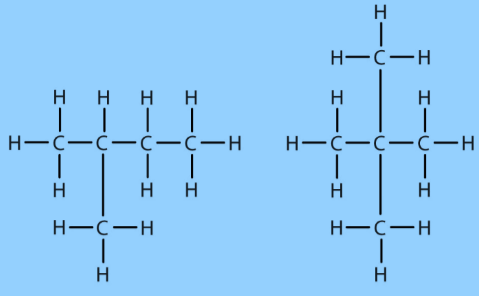
Carbon Compounds based on the structure
Carbon Compounds can be classified as straight-chain compounds, branched-chain compounds and cyclic compounds. They are represented as -
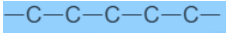
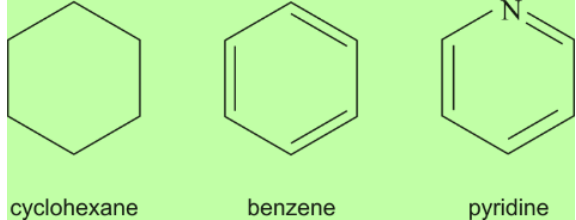
Functional Groups
One of the hydrogen atoms in hydrocarbons can be replaced by other atoms according to their valencies.The atoms which decide the properties of the carbon atoms are known as Functional Groups.
Example, Cl, Br, -OH, Aldehyde, Ketone, Carboxylic Acid etc.
Homologous series
A series of organic compounds having the same functional group and chemical properties and in which the successive members differ by CH2 unit or 14 mass units is known as Homologous series.
Characteristic of homologous series are:
- The successive members in homologous series differ by CH2 unit or 14 mass unit.
- All compounds of a homologous series have the same functional group.
- All compounds of homologous series show similar chemical properties due to the addition of same kind of functional group throughout the chain.
For example,
Homologous series of alkane is CH4 (Methane), C2H6 (Ethane), C3H8 (Propane),..
Nomenclature of Carbon Compounds
- First of all, identify the number of carbon atoms in compounds. And in it identify the longest chain
- Then the functional group can be indicated by suffix or prefix.
- Cyclic hydrocarbon is designated by the prefix cyclo.
- If there are two or more different substituents they are listed in alphabetical order
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given
5. Soaps and Detergents
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Soaps & Detergents
Sodium or potassium salt of a carboxylic acid is known as Soap. They work most effectively in soap water. Detergents are sulfonates or ammonium salt of a long chain of carboxylic acid. They can work effectively on soft as well as hard water.
Cleansing actions of Soaps & Detergents
The cleansing action of soaps and detergents is due to the ability to minimize the surface tension of water, to emulsify oil or grease and hold them in a suspension of water. When soap dissolves in water, it forms soap anions and soap cations. The hydrophobic part of soaps and detergents are soluble in grease and the hydrophilic part is soluble in water.
Soaps & Micelle Formation
- When dirt and grease are mixed with soap water, soap molecules arrange them in tiny clusters known as Micelle.
- The hydrophilic part sticks to the water and forms the outer surface of the micelle and the hydrophobic part binds to oil and grease.
- Soap micelles help to dissolve dirt and grease in water and clothes gets cleaned.
- soap is a mixture of micelles.
- The magnesium and the calcium salts present in the hard water react with soap molecules to form in soluble product called scum.
- This scum create difficulty in cleansing action.
- By use of detergent, insoluble scum is not formed with hard water and clothes get cleaned effectively.

4. Carbon Compounds- Ethanol and Ethanoic Acid
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Properties of Ethanol
Ethanol is a volatile liquid with a low melting point. It reacts with sodium to form sodium ethoxide.

This reaction is used to test the presence of ethanol by the evolution of hydrogen gas.
Dehydration of ethanol in presence of hot sulphuric acid forms alkene.
![]()
Properties of Ethanoic acid
Ethanoic acid is a colourless liquid. When pure ethanoic acid freezes like ice, it is known as Glacial Acetic Acid. It is formed at a temperature of about 16.6 degrees centigrade
Ethanoic Acid/Acetic acid when reacts with ethanol forms an ester. Ester can be identified by its sweet smell.
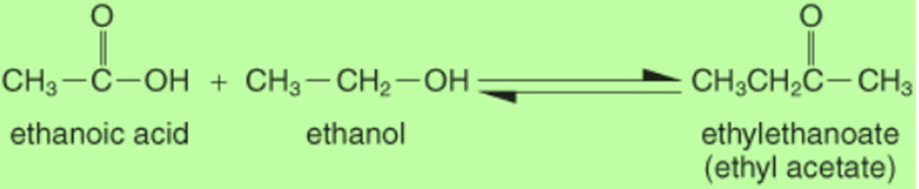
The reaction of esters with a strong base is used to form soap. This is known as Specification. Acetic acid also reacts with a strong base to form sodium acetate and water.
NaOH + CH3COOH + CH3COONa + H2O
3. Chemical Properties of Carbon Compounds
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chemical properties of Carbon Compounds
Combustion
Carbon along with its compound is used as a fuel as it burns in presence of oxygen to release energy. Saturated hydrocarbons produce blue and non-sooty flame whereas unsaturated hydrocarbons produce a yellow sooty flame.
CH4 + 2O2 → CO2 + 2H2O
Oxidation
Alcohol can be oxidised to aldehydes whereas aldehydes, in turn, can be oxidised to carboxylic acid. Oxidising agents such as potassium permanganate can be used for oxidation.

Addition Reaction
Hydrogenation of vegetable oil is an example of an additional reaction. Addition of hydrogen in presence of catalysts such as nickel or palladium. This converts the oil into ghee.
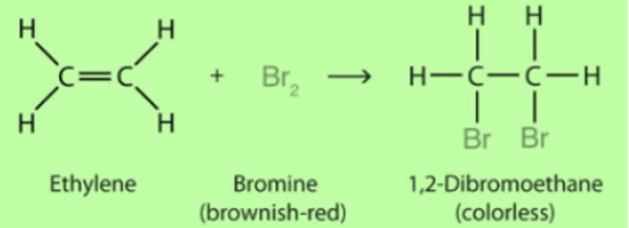
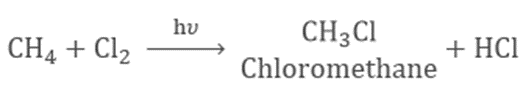
Substitution Reaction
When one atom in hydrocarbon is replaced by chlorine, bromine, etc. this is known as a Substitution Reaction.
2. Mendeleev's Periodic Table
- Books Name
- SonikaAnandAcademy Science Book
- Publication
- SonikaAnandAcademy
- Course
- CBSE Class 10
- Subject
- Science
Periodic classification of elements
Sample paper
- What is the mendeleev periodic law?
- What is modern periodic law?
- What is the name given to the horizontal rows and vertical column
- What are the drawbacks of the mendeleev periodic law
- How many groups and periods are found in the mendeleev periodic law
- Why noble gases are not present in the mendeleev periodic table
- How many groups and periods are found in the long form of table
- What is ionization enthalpy why the second ionization enthalpy of alkali metals is greater than the first ionization enthalpy
- Why the atomic radius of chlorine is less than atomic radius of sodium
- What is electronegativity how does electronegativity variates in periodic table from moving top to bottom
- Why hydrogen is placed at the top of the periodic table
- Who discover the atomic number
- What are diagonal elements
- What is electron affinity how does it changes when we move from left to right in the periodic table
- Why noble gases do not react with any other elements
If you want the answer of the above questions and recorded lectures or live classes for doubt sessions just WhatsApp at the number 7007820783
2. Mendeleev's Periodic Table
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Mendeleev’s Periodic Table
Mendeleev’s Periodic Law:
Physical and chemical properties of elements are the periodic function of their atomic masses.
Mendeleev’s periodic table is based on the chemical properties of elements.
Mendeleev’s periodic table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’.
Achievements of Mendeleev’s Periodic Table: 63 elements were known at the time of classification.
- Elements with similar properties could be grouped together.
- Mendeleev left some gaps in his periodic table. Mendeleev boldly predicted the existence of some elements that had not been discovered at that time.
- Noble gases discovered, could be placed without disturbing the existing order.
Remember
Scandium, gallium and germa-nium have properties similar to Eka-boron, Eka-aluminium and Eka-silicon respectively.
Limitations of Mendeleev’s Classification
- The position of hydrogen in the table was not certain because it could be placed in the group of alkali metals as well as in halogens.
- Isotopes of elements were placed in the same position in the table though according to their atomic masses, they should have been placed in different positions.
- Certain elements of higher atomic mass proceed those with lower atomic mass. For example, tellurium (atomic mass 127.6) precedes iodine (atomic mass 126.9). Iodine was placed after tellurium though it had lower atomic mass because Iodine had properties similar to bromine and not selenium.
3. Modern Periodic Table
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
The Modern Periodic Table:
Periodicity. The properties which reoccur after a regular intervals in a periodic table are called periodic properties and the phenomenon is called periodicity of element.
Henry Moseley (1913) exhibited that the atomic number of an element is a more fundamental property than its atomic mass. Mendeleev’s periodic law was modified and atomic number was adopted as the basis of the modern periodic table.
The Modern Periodic Law states that:
The physical and chemical properties of the elements are the periodic function of their atomic numbers.
It means that if the elements are arranged in order of increasing atomic numbers, the elements with similar properties recur after regular intervals.
Many new forms of periodic table have been proposed in recent times with modern periodic law as the guiding principle, but the general plan of the table remains the same as proposed by Mendeleev. The most commonly known periodic table is the Long form of the periodic table.Few Points to remember:
- Modern periodic table contains 18 vertical columns known as groups and 7 horizontal rows known as periods.
- Elements in a group have the same number of valence electrons.
- Number of the shells increases as we go down the group.
- Elements in a period have same number of shells.
- Number of elements placed in a particular period depends upon the fact that how electrons are filled into various shells.
- Maximum number of electrons that can be accommodated in a shell depends on the formula 2n2 where n is the number of the given shell. For example, in K shell the number of electrons is 2 x (1)2 = 2 in the first period, L shell – 2 x (2)2 = 8 elements in the second period.
- Position of the element in the periodic table tells about its reactivity.
Trends in the Modern Periodic Table
Valency: Number of valence electrons present in the outermost shells. Valency remains the same down a group but changes across a period.
Atomic Size: Atomic size refers to radius of an atom.
Atomic size or radius decreases in moving from left to right along a period due to increase in nuclear charge. Atomic size increases down the group because new shells are being added as we go down the group.
Metallic Character: Metallic character means the tendency of an atom to lose electrons.
Metallic character decreases across a period because the effective nuclear charge increases, that means the tendency to lose electrons decreases. Metals are electropositive as they tend to lose electrons while forming bonds.

 Carrier Point
Carrier Point
