- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 5
Periodic Classification of Elements
Introduction
At present, 118 elements are known to us.
All these have different properties. Out of these 118, only 98 are naturally occurring.
These elements have different characteristic properties, so it is very difficult to study these elements individually.
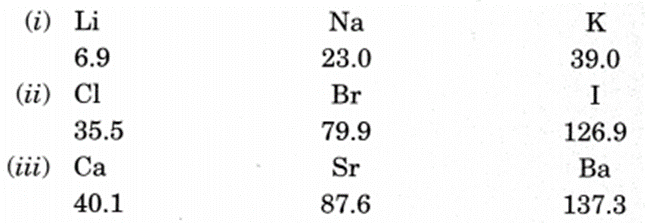
Dobereiner’s Triads (1817):
He identified some triads (groups having three elements).
Dobereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was the average of the atomic masses of the other three elements. For example, Dobereiner could identify only three triads from the elements known at that time. Hence, this system of classification into triads was not found to be useful.
Newland’s Law of Octaves (1866):
- He arranged the known elements in the order of increasing atomic masses.
- He found that every eighth element had properties similar to that of the first like the musical note. It is known as Newlands law of octaves.
Limitations
- Law of octaves was applicable only upto calcium.
- Discovery of Noble gases disturbed the octaves.
- Octaves worked well only for lighter elements.
- Properties of new elements could not fit in it.

 Vaishnav Publication
Vaishnav Publication
