Chemical change – one or more new substances with new physical and chemical properties are formed.
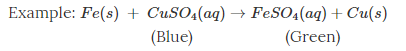
Here, when copper sulphate reacts with iron, two new substances, i.e., ferrous sulphate and copper are formed.
A chemical equation is a statement or representation that describes a chemical reaction in terms of
symbol and formulae.
➢ A chemical reaction is the transformation of chemical substance called reactants into another chemical
substance called products. In a chemical reaction, only rearrangement of atoms takes place.
➢ The substances which take part in a chemical reaction are called reactants. The reactants are written
on the left hand side. The new substances produced as a result of chemical reaction are called
products. The products are written on the right hand side.
Some of the symbols used in a chemical Equation are :
(a) Solids (s)
(b) Liquids (l)
(c) Gases (g)
(d) Aqueous solutions (aq)
(e) Gas released as a product (↑)
(f) Precipitate formed in the reaction (↓)
(g) Direction of reaction (→)
➢ Steps to balance a chemical equation :
(a) Write word equation.
(b) Then write skeletal chemical equation.
(c) Enclose the formula in the boxes.
(d) List the number of atoms of different elements present in the unbalanced equation.
(e) Start balancing with the compound that contains the maximum number of atoms.
(f) Start balancing other atoms.
(g) Check the correctness of the balanced equation.
- Books Name
- SonikaAnandAcademy Science Book
- Publication
- SonikaAnandAcademy
- Course
- CBSE Class 10
- Subject
- Science
NCERT Solutions -Class 10 -Chemical Reactions and Equations
Chemical Reaction
When two or more substance react with each other to form one or more new substance this is called chemical reaction
The substances which reacts with each other in a chemical reaction are known as reactants they are generally written on the left hand side of the equation
The substances which are formed in a chemical reaction are known as product they are generally written on the right hand side of the equation
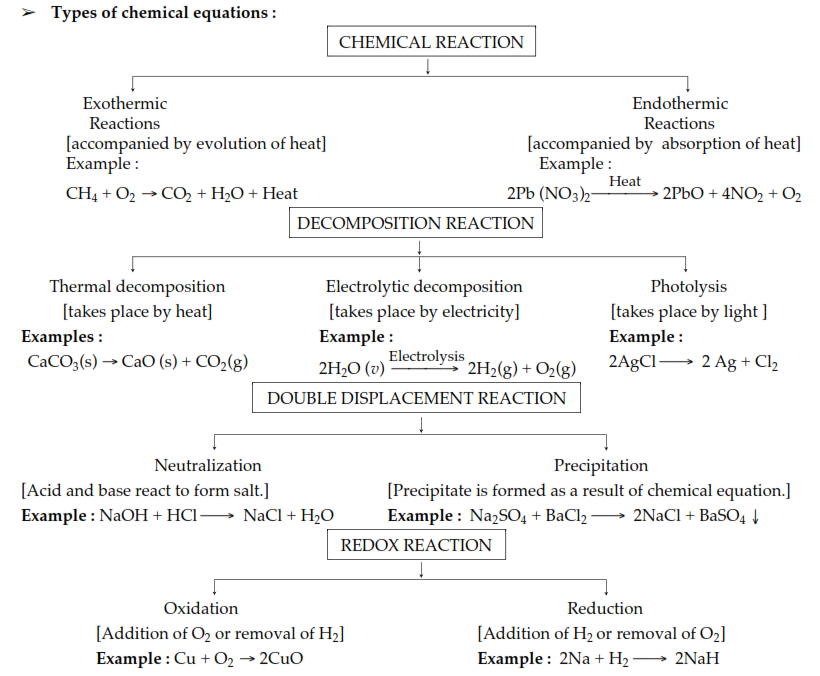
Chemical reactions can be of different types
1) Combination reaction-- when two or more substances combine together to form a new substance is called combination reaction
Symbolically
A+B ------C
Two substances A and B are combined together to form a new substance C
Example
H2+Cl2------2HCl
2) Decomposition reaction-- when a substance decomposes into two or more substances is called decomposition reaction
Symbolically
C---A+B
The compound C decomposes to two compounds A and. B
Example
ZnCO3------Zn + CO2
The composition of a compound can be done by either of the methods
--- by thermal decomposition
--- by electrolysis
-----by photolysis reaction
3) Displacement Reaction
When an element of a compound displaces by another element during a chemical reaction is known as displacement reaction
Symbolically
AB+C-----AC+B
In above equation element C displaces element B of the compound AB so this type of reaction is known as displacement reaction
2HCl+Mg------MgCl2 + H2
4) Double displacement reaction
When elements of compounds and exchanged each other in a chemical reaction what is known as double displacement reaction
Symbolically
AB+CD-----AD+BC
In the above reaction elements of the compound AB and elements of the compounds CD are exchange together to form a compounds AD and BC
2KBr+BaI2-------2KI. + BaBr2
Types of reactions positive ion of one compound reacts with negative ions of another compound similarly in negative ions of the first compound combines with positive
Redox Reaction
The reaction in which there is a oxidation and reduction both occurs is known as red oxide reaction in this type of reaction there is gain of electron in one element and loss of electrons in an atom
The element which gains electrons undergoes oxidation reaction it can also be defined as addition of oxygen is known as oxidation reaction
The element which loses electron undergoes reduction reaction or in other words addition of hydrogen is known as reduction reaction
The substance which undergoes oxidation is known as reducing agent
The substance which undergoes reduction is known as oxidising agent
Limiting Reagent
The substance which consumes completely in a chemical reaction is called limiting reagent generali on limiting reagent decide the extent of a chemical reaction
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 1
Chemical reactions and Equations
Chemical Reaction: The transformation of chemical substance into another chemical substance is known as Chemical Reaction. For example: Rusting of iron, the setting of milk into curd, digestion of food, respiration, etc.
In a chemical reaction, a new substance is formed which is completely different in properties from the original substance, so in a chemical reaction, a chemical change takes place. Only a rearrangement of atoms takes place in a chemical reaction.
The substances which take part in a chemical reaction are called reactants.
The new substances produced as a result of a chemical reaction are called products.
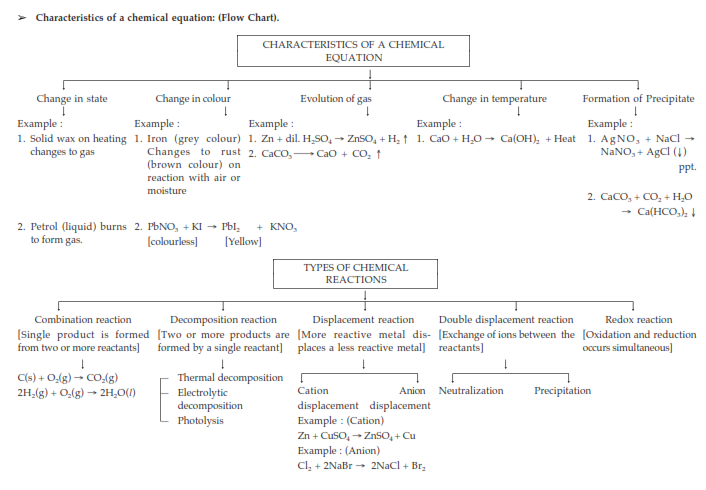
Characteristics of Chemical Reactions:
(i) Evolution of gas: The chemical reaction between zinc and dilute sulphuric acid is characterised by the evolution of hydrogen gas.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) ↑
(ii) Change in Colour: The chemical reaction between citric acid and purple coloured potassium permanganate solution is characterised by a change in colour from purple to colourless.
The chemical reaction between sulphur dioxide gas and acidified potassium dichromate solution is characterized by a change in colour from orange to green.
(iii) Change in state of substance: The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas (because the wax is a solid, water formed by the combustion of wax is a liquid at room temperature whereas, carbon dioxide produced by the combustion of wax is a gas). There are some chemical reactions which can show more than one characteristic.
(iv) Change in temperature: The chemical reaction between quick lime water to form slaked lime is characterized by a change in temperature (which is a rise in temperature).
The chemical reaction between zinc granules and dilute sulphuric acid is also characterised by a change in temperature (which is a rise in temperature).
(v) Formation of precipitate: The chemical reaction between sulphuric acid and barium chloride solution is characterised by the formation of a white precipitate of barium sulphate.
BaCl2(aq) + H2SO4(aq) → BaSO4(s) (ppt) + 2HCl(aq)
Chemical Equation:
Representation of chemical reaction using symbols and formulae of the substances is called Chemical Equation.
Example: A + B → C + D
In this equation, A and B are called reactants and C and D are called the products. The arrow shows the direction of the chemical reaction. Condition, if any, is written generally above the arrow.
When hydrogen reacts with oxygen, it gives water. This reaction can be represented by the following chemical equation:
Hydrogen + Oxygen → Water H2 + O2 → H2O
In the first equation, words are used and in second, symbols of substances are used to write the chemical equation. For convenience, the symbol of substance is used to represent chemical equations.
A chemical equation is a way to represent the chemical reaction in a concise and informative way.
A chemical equation can be divided into two types: Balanced Chemical Equation and Unbalanced Chemical Equation.
(a) Balanced Chemical Equation: A balanced chemical equation has the number of atoms of each element equal on both sides.
Example: Zn + H2SO4 → ZnSO4 + H2
In this equation, numbers of zinc, hydrogen and sulphate are equal on both sides, so it is a Balanced Chemical Equation.
According to the Law of Conservation of Mass, mass can neither be created nor destroyed in a chemical reaction. To obey this law, the total mass of elements present in reactants must be equal to the total mass of elements present in products.
(b) Unbalanced Chemical Equation: If the number of atoms of each element in reactants is not equal to the number of atoms of each element present in the product, then the chemical equation is called Unbalanced Chemical Equation.
Example: Fe + H2O → Fe3O4 + H2
In this example, a number of atoms of elements are not equal on two sides of the reaction. For example; on the left-hand side only one iron atom is present, while three iron atoms are present on the right-hand side. Therefore, it is an unbalanced chemical equation.
Steps to form balanced chemical equation
To show how to balance the equation, the following equation is used-
Fe + H2O → Fe3O4 + H2
Step 1: First of all, draw the boxes around each formula as shown below-
![]()
Step 2: Find out the number of atoms of each element. For Example, on reactant side, 1 for Fe, 2 H, and 1 O and on product side we have, 3 for Fe, 4 for O and 2 for H.
Step 3: Start to balance the equation with the compound having maximum number of atoms. While balancing does not alter the formula of the compound.
Step 4: One by one balance each element on reactant and product side.
![]()
Step 5: After balancing number of atoms on both the side of the equation, finally check the correctness of the balanced equation.
![]()
Step 6: then write the symbols of the physical state of reactants and products as shown below-
3Fe(s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g)
This above equation represents the balanced equation.

 Carrier Point
Carrier Point
