1. Bonding in Carbon
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 4
Carbon & its Compounds
Introduction
Covalent Bond: The atomic number of carbons is 6. Its electronic configuration is 2, 4. It requires, 4 electrons to achieve the inert gas electronic configuration. But carbon cannot form an ionic bond.
It could gain four electrons forming C4- cation. But it would be difficult for the nucleus with six protons to hold on to ten electrons.
It could lose four electrons forming C4+ cations. But it requires a large amount of energy to remove four electrons.
Thus, carbon overcomes this problem by sharing of its valence electrons with other carbon atoms or with atoms of other elements.
The bond formed by mutual sharing of electron pairs between two atoms in a molecule is known as Covalent Bond.
Types of Covalent Bond:
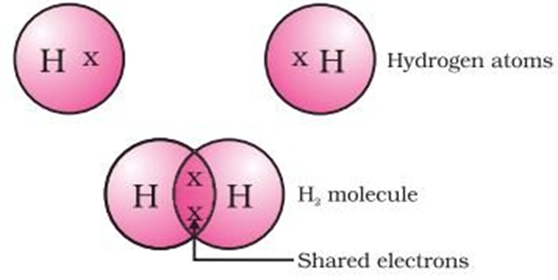
Single Covalent Bond: When a single pair of electrons are shared between two atoms in a molecule. For example; F2, Cl2, H2 etc.
The atomic number of hydrogen is 1. Hence it has one electron in its K shell and it requires one more electron to fill the K shell. So, two hydrogen atoms share their electrons to form a molecule of hydrogen, H2.
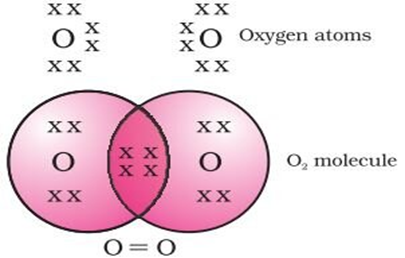
Double Covalent Bond: When two pairs of electrons are shared between two atoms in a molecule. For example; O2, CO2 etc.
The atomic number of oxygen is 8. So it has six electrons in its L shell and it requires two more electrons to complete its octet. So each atom of oxygen shares two electrons with another atom of oxygen. The two electrons contributed by each oxygen atom give rise to a double bond between the two atoms.
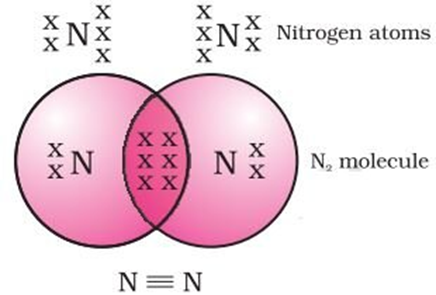
Triple Covalent Bond: When three pairs of electrons are shared between two atoms in a molecule. For example; N2 etc.
Nitrogen has the atomic number 7. So, it has five electrons in its L shell and it requires three more electrons to complete its octet. So, in order to attain an octet, each nitrogen atom in a molecule of nitrogen contributes three electrons giving rise to three shared pairs of electrons that results in a triple bond between the two atoms.
Allotropes of Carbon
- Different forms of an element that have same chemical properties but different physical properties are known as Allotropes.
- There are three allotropes of carbon - Diamond, graphite and Fullerene
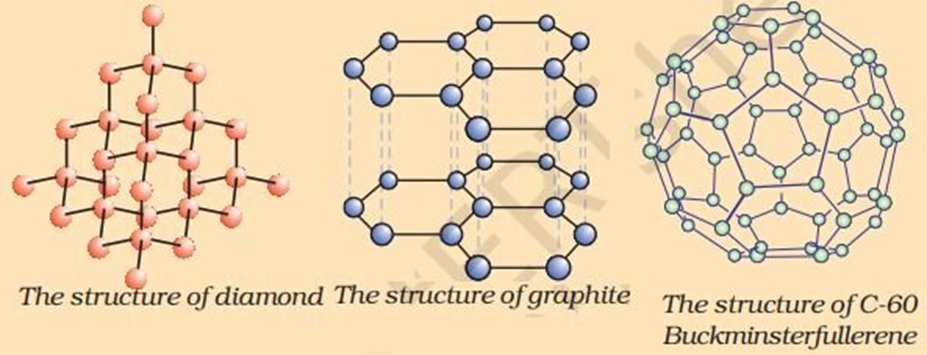
(i) Diamond
In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure.
→ It is the hardest substance known.
→ It has high melting point.
→ It is a bad conductor of electricity.
→ It is used in making jewellery. It is also used in cutting and drilling tools.
(ii) Graphite
→ Each carbon atom is bonded with other three carbon atoms in the same plane forming hexagonal rings.
→ These rings are stacked over each other through weak Van der Waals forces.
→ Graphite is also a very good conductor of electricity.
→ It is used as dry lubricant.
→ It is used in making pencil lead.
(iii) Fullerene
→ C60, also known as Buck minster fullerene, is the most common form of fullerenes.
→ It has carbon atoms arranged in the shape of a football.
→ It consists of 60 carbon atoms arranged in 12 pentagons and 20 hexagons.
Versatile nature of carbon
Carbon is included in ten formations of a variety of compounds. This is due to the following two reasons:
(i) Catenation: Carbon has the unique ability to form covalent bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation.
Carbon exhibits catenation property due to the following reasons:
→ Size of the carbon atom is very small. This enables its nucleus to hold the shared pair of electrons strongly.
→ The carbon-carbon bond is quite strong.
(ii) Tetravalent Nature: Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
2. Nature of Carbon Compounds
- Books Name
- SonikaAnandAcademy Science Book
- Publication
- SonikaAnandAcademy
- Course
- CBSE Class 10
- Subject
- Science
Carbon and its compounds
Sample paper
- 1)What is catenation
- 2)What are isomers write the isomers of butane
- 3) What is the mean by homologous series explain with example
- 4) What is the difference between saturated and unsaturated compounds give two examples of each
- 5) How many number of bonds are present in alkane alkene and alkyne
- 6) What is the general formula of alkyl group and cycloalkanes
- 7) Write a formula of
- cyclopentane
- Propyne
- butanol
- Benzene
- 8) What are soaps explain its structure and cleansing action of soap
- 9) what do the detergent forms when they mix and water and what is the difference between detergent and soap
- 10) What are the products formed
- When the flame burns with blue flame and
- when the bus with yellow frame
11)
What is difference between combustion and oxidation
12) what do you mean by saponification explain with reaction
13) what is the eastrification give the chemical reaction and what are the products formed
14) what are addition and substitution reactions give one example of each
15) explain the tetravalency of carbon what is the reason why there are lot of carbon compounds are made by the carbon
2. Nature of Carbon Compounds
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Hydrocarbons
Compounds of carbon and hydrogen are known as hydrocarbons.
For example - Methane (CH4), Ethane (C2H6), etc.
Saturated hydrocarbons: These hydrocarbons have all carbon-carbon single bonds. These are known as alkanes.
General formula = CnH2n+2
where n = number of carbon atoms
Unsaturated hydrocarbons: These hydrocarbons have at least one carbon-carbon double or triple bond.
→ Hydrocarbons with carbon-carbon double bond are known as alkenes. General formula = CnH2n
→ Hydrocarbons with carbon-carbon triple bond are known as alkynes. General formula = CnH2n-2
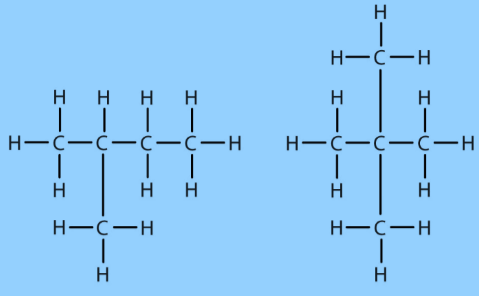
Carbon Compounds based on the structure
Carbon Compounds can be classified as straight-chain compounds, branched-chain compounds and cyclic compounds. They are represented as -
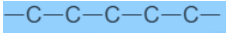
Functional Groups
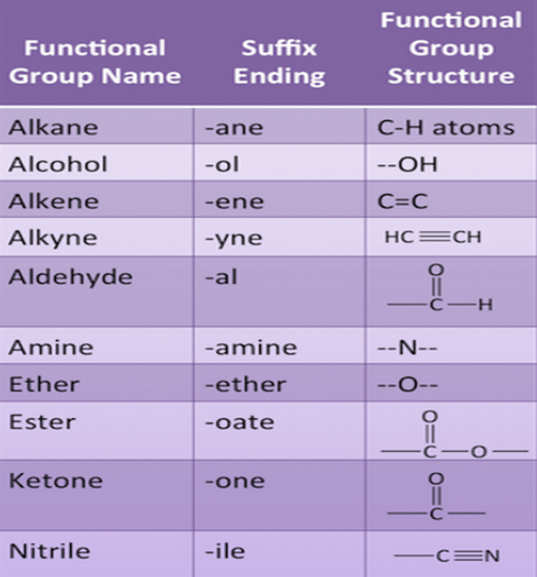
One of the hydrogen atoms in hydrocarbons can be replaced by other atoms according to their valencies.The atoms which decide the properties of the carbon atoms are known as Functional Groups.
Example, Cl, Br, -OH, Aldehyde, Ketone, Carboxylic Acid etc.
Homologous series
A series of organic compounds having the same functional group and chemical properties and in which the successive members differ by CH2 unit or 14 mass units is known as Homologous series.
Characteristic of homologous series are:
- The successive members in homologous series differ by CH2 unit or 14 mass unit.
- All compounds of a homologous series have the same functional group.
- All compounds of homologous series show similar chemical properties due to the addition of same kind of functional group throughout the chain.
For example,
Homologous series of alkane is CH4 (Methane), C2H6 (Ethane), C3H8 (Propane),..
Nomenclature of Carbon Compounds
- First of all, identify the number of carbon atoms in compounds. And in it identify the longest chain
- Then the functional group can be indicated by suffix or prefix.
- Cyclic hydrocarbon is designated by the prefix cyclo.
- If there are two or more different substituents they are listed in alphabetical order
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given
5. Soaps and Detergents
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Soaps & Detergents
Sodium or potassium salt of a carboxylic acid is known as Soap. They work most effectively in soap water. Detergents are sulfonates or ammonium salt of a long chain of carboxylic acid. They can work effectively on soft as well as hard water.
Cleansing actions of Soaps & Detergents
The cleansing action of soaps and detergents is due to the ability to minimize the surface tension of water, to emulsify oil or grease and hold them in a suspension of water. When soap dissolves in water, it forms soap anions and soap cations. The hydrophobic part of soaps and detergents are soluble in grease and the hydrophilic part is soluble in water.
Soaps & Micelle Formation
- When dirt and grease are mixed with soap water, soap molecules arrange them in tiny clusters known as Micelle.
- The hydrophilic part sticks to the water and forms the outer surface of the micelle and the hydrophobic part binds to oil and grease.
- Soap micelles help to dissolve dirt and grease in water and clothes gets cleaned.
- soap is a mixture of micelles.
- The magnesium and the calcium salts present in the hard water react with soap molecules to form in soluble product called scum.
- This scum create difficulty in cleansing action.
- By use of detergent, insoluble scum is not formed with hard water and clothes get cleaned effectively.

4. Carbon Compounds- Ethanol and Ethanoic Acid
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Properties of Ethanol
Ethanol is a volatile liquid with a low melting point. It reacts with sodium to form sodium ethoxide.

This reaction is used to test the presence of ethanol by the evolution of hydrogen gas.
Dehydration of ethanol in presence of hot sulphuric acid forms alkene.
![]()
Properties of Ethanoic acid
Ethanoic acid is a colourless liquid. When pure ethanoic acid freezes like ice, it is known as Glacial Acetic Acid. It is formed at a temperature of about 16.6 degrees centigrade
Ethanoic Acid/Acetic acid when reacts with ethanol forms an ester. Ester can be identified by its sweet smell.
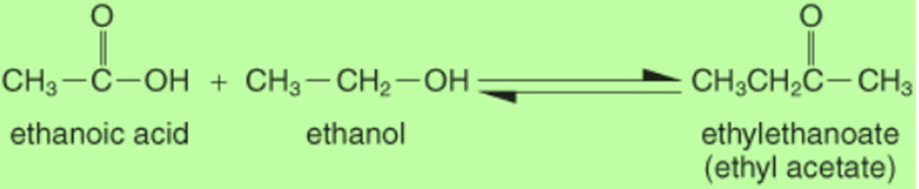
The reaction of esters with a strong base is used to form soap. This is known as Specification. Acetic acid also reacts with a strong base to form sodium acetate and water.
NaOH + CH3COOH + CH3COONa + H2O
3. Chemical Properties of Carbon Compounds
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chemical properties of Carbon Compounds
Combustion
Carbon along with its compound is used as a fuel as it burns in presence of oxygen to release energy. Saturated hydrocarbons produce blue and non-sooty flame whereas unsaturated hydrocarbons produce a yellow sooty flame.
CH4 + 2O2 → CO2 + 2H2O
Oxidation
Alcohol can be oxidised to aldehydes whereas aldehydes, in turn, can be oxidised to carboxylic acid. Oxidising agents such as potassium permanganate can be used for oxidation.

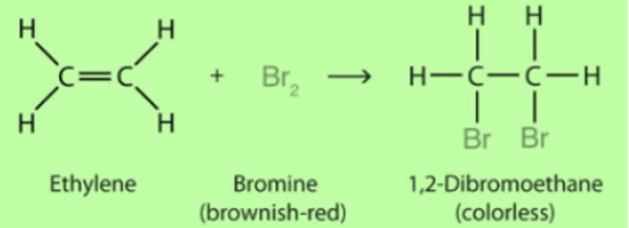
Addition Reaction
Hydrogenation of vegetable oil is an example of an additional reaction. Addition of hydrogen in presence of catalysts such as nickel or palladium. This converts the oil into ghee.
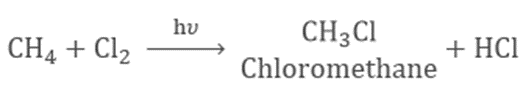
Substitution Reaction
When one atom in hydrocarbon is replaced by chlorine, bromine, etc. this is known as a Substitution Reaction.

 Vaishnav Publication
Vaishnav Publication
