1. Physical Properties of Metals and Non-Metals
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chapter 3
Metals & Non metals
Introduction
Elements are classified as metals and non-metals based on different properties. The properties of metals and non-metals are given in the form of the table below-
Physical Properties of Metals
(i) They are lustrous, i.e., they have a shiny surface.
(ii) All metals are solids except mercury which is liquid at room temperature.
(iii) Metals are ductile which means they can be drawn into wires.
(iv)Metals are malleable which means they can be beaten into sheets. The only exception is mercury.
(v) They are good conductors of heat and electricity except lead and mercury.
(vi)They have high density and high melting point except alkali metals like lithium, sodium, potassium is so soft that they can be cut with a knife.
Physical Properties of Non-metals
(i) They are non-lustrous and have dull appearance except graphite and iodine that are lustrous.
(ii) They are generally soft, except diamond.
(iii) They are non-ductile.
(iv)They are non-malleable.
(v) They are poor conductors of heat and electricity except graphite which is a good conductor of electricity
(vi)They have low density and low melting point except diamond which has high melting point.
2. Chemical Properties of Metals
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Chemical properties of Metals
Metals react with air or oxygen to form metal oxide.
For Example, Copper reacts with oxygen to form copper oxide.
Metal + O2 → Metal oxide
2Cu + O2 → 2CuO
4Al + 3O2 → 2Al2O3
Oxides of metals can react with both acids and bases to produce salt and water. Such oxides are known as Amphoteric Oxides.
Al2O3 + 6HCl → 2AlCl3 + H2O

Metals also react with water to form metal oxide. Metal oxide in turn can react with water to form metal hydroxide.
For Example
2Na + 2H2O → 2NaOH + 1H2
2Al + 3H2O → Al2O3 + 3H2
Metals also react with dilute acids to form salt and hydrogen.
For example, magnesium reacts with dilute hydrochloric acid to form magnesium chloride and hydrogen.
Metal + Acid → Metal Salt + Hydrogen
Mg + 2HCl → MgCl2 + H2
Reaction of metals with solutions of other metal salts
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Fe + CuSO4 → FeSO4 + Cu
Cu + 2AgNO3 → Cu (NO3) + 2Ag
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Fe + CuSO4 → FeSO4 + Cu
Cu + 2AgNO3 → Cu (NO3) + 2Ag
Special cases
- Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
- At ordinary temperature, the surfaces of metals such as magnesium, aluminum, zinc, lead, etc., are covered with a thin layer of oxide. The protective oxide layer prevents the metal from further oxidation.
- Iron does not burn on heating but iron filings burn vigorously when sprinkled in the flame of the burner.
- Copper does not burn, but the hot metal is coated with a black coloured layer of copper (II) oxide.
- Silver and gold do not react with oxygen even at high temperatures.
Chemical properties of Non-metals
Non-metals react with oxygen to form non-metal oxide.
Non-metal + Oxygen → Non-metal oxide
C + O2 → CO2
Non-metals do not react with water and acids to evolve hydrogen gas.
Non-metals can react with salt solution; the more reactive element will displace the less reactive non-metal.
2 NaBr (aq) + Cl2(aq) → 2NaCl (aq) + Br2 (aq)
Non-metals can also react with hydrogen to form hydrides.
H2(g) + S(l) → H2S(g)
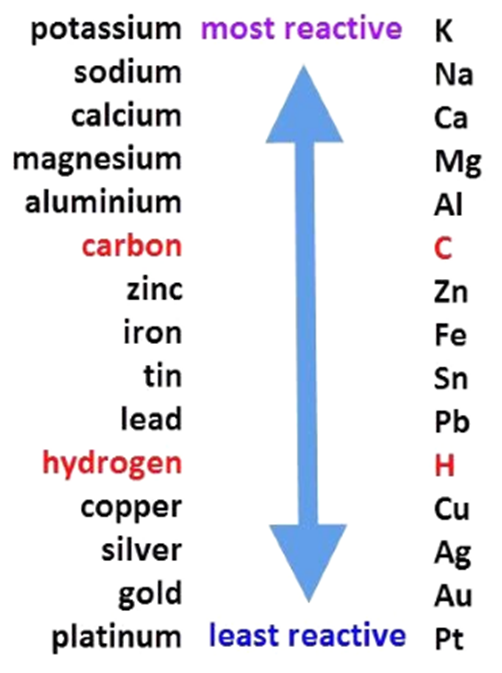
Reactivity Series
The series in which metals are arranged in the decreasing order of reactivity is known as the Reactivity Series.
3. Metals and Non-Metals Reactions
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
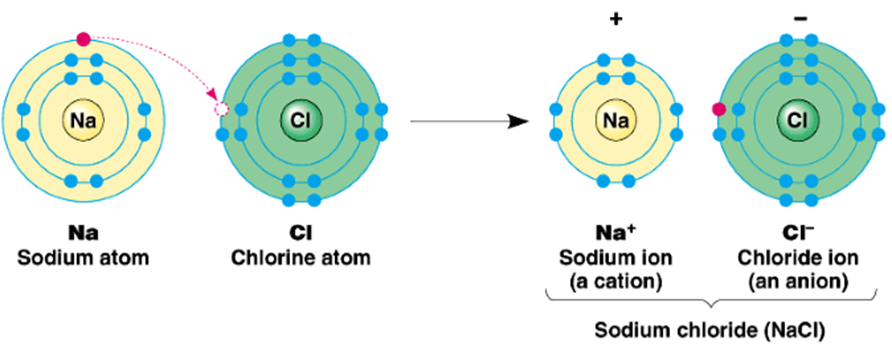
Ionic Compounds
Compounds formed due to the transfer of electrons from a metal to a non-metal are known as Ionic Compounds.
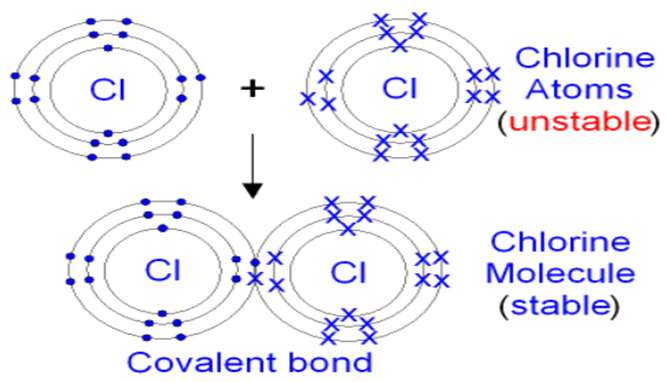
Covalent Bond
A bond is formed by the sharing of electrons between the two atoms. They share their valence electrons to gain stability.
Properties of Ionic Compounds
- They are generally hard and solid.
- They have a high melting and boiling point.
- They are soluble in water but insoluble in inorganic solvents such as ether etc.
- They are conductors of electricity in molten and solution states.
4. occurrence of Metals
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Occurrence of Metals
Metals are generally found in the earth’s crust.
Elements or compounds which occur naturally in earth crust are known as Minerals. Minerals from which pure metals can be extracted are known as Mineral Ores.
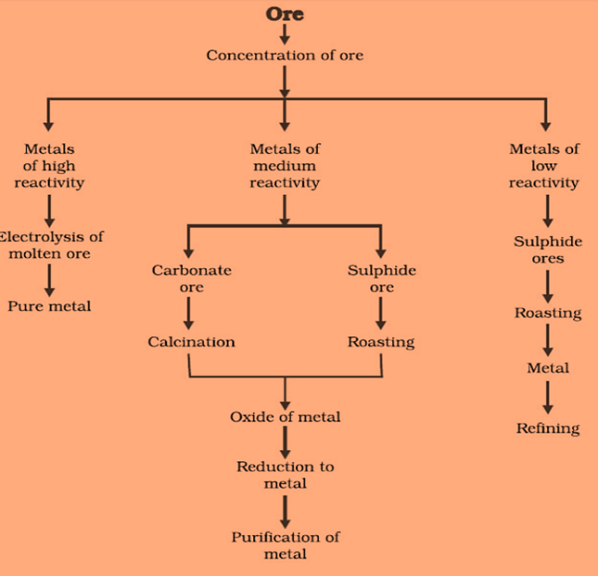
Steps for extraction of metals from its ore are:
- The first step is the enrichment of the ore
- The second step includes extraction of metals
- Third steps involve refining of metal
Gangue - Ores contain different impurities in it such as sand, soil etc. These impurities are known as Gangue.
Extracting Metals which are low in activity series
Metals which are low in the activity series are unreactive. The oxides of such metals can be reduced to metals by heating alone. For Example, Cinnabar (HgS)

Extracting Metals in the middle of the Activity Series
These metals are moderately reactive. They exist as sulphides or carbonates in nature. Before reduction, metal sulphides and carbonates must be converted into metal oxides.by heating strongly in the presence of excess air, this is known as Roasting. Carbonate ores are converted into oxides by heating in limited air. This is known as Calcination.
Roasting

Calcination

Reduction-metal oxides can be reduced to metals using a reducing agent such as Carbon.
Extracting metals towards the top of the activity series
The metals are highly reactive. They cannot be obtained by heating. For Example, Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
At cathode Na+ + e- → Na
At anode 2Cl- → Cl2 + 2e-
Refining of metals
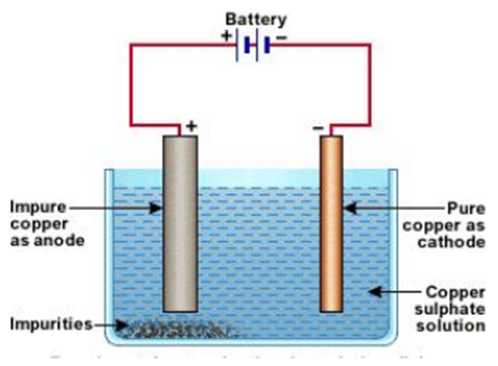
Refining of impure metal is done using electrolytic refining. Impure copper is used as anode and a strip of pure copper is used as Cathode. Acidified copper sulphate is used as an electrolyte. When an electric current is passed through this, impure metal from the anode gets deposited in the electrolyte solution, whereas pure metal from the electrolyte is deposited at the cathode.
Deposition of insoluble residue formed from the dissolution of the anode during commercial electrolysis.
5. Corrosion
- Books Name
- Iti Shree Science Book
- Publication
- Vaishnav Publication
- Course
- CBSE Class 10
- Subject
- Science
Corrosion
When exposed to moist air for a long period of time, metals become corroded. This is known as Corrosion. For Example, Silver reacts with moist air and becomes black in colour due to silver sulphide coating.
Iron + oxygen → Iron (III) oxide
Fe + O2 → Fe2O3
Prevention of Corrosion
- Rusting of iron can be prevented by oiling, galvanising, painting, greasing etc.
- To protect steel and iron from rusting, a thin layer of zinc is coated on them, this is known as Galvanization.

 Vaishnav Publication
Vaishnav Publication
